Abstract
-
Objectives:
- This study was performed to investigate the correlation between subjective residual dizziness and objective postural imbalance after successful canalith repositioning procedure (CRP) in benign paroxysmal positional vertigo (BPPV) by using questionnaires and modified Clinical Test of Sensory Integration and Balance (mCTSIB).
-
Methods:
- A total of 31 patients with BPPV were included prospectively in the study. All included patients were successfully treated after initial CRP and their symptoms and nystagmus disappeared. Two weeks after CRP, all patients were asked to fill out the questionnaire including both Dizziness Handicap Inventory (DHI) and visual analog scale (VAS). We also conducted mCTSIB 2 weeks after CRP. We divided patients into two groups according to VAS: RD (residual dizziness) group, VAS>0; non-RD group, VAS=0. We compared age, number of CRP, rates associated with three semicircular canals, DHI score and mCTSIB results between two groups. In addition, we analyzed the correlation between DHI score and mCTSIB results.
-
Results:
- There were no significant differences in age, number of CRP, and rates associated with three semicircular canals between the two groups. RD group showed significantly higher DHI score and abnormal mCTSIB results than the non-RD group (p<0.05). DHI score and the number of abnormal mCTSIB showed a statistically significant correlation.
-
Conclusions:
- We demonstrated the correlation between DHI score and mCTSIB after successful CRP for BPPV. It also represents that subjective residual dizziness is correlated with objective postural imbalance even after successful CRP. Therefore, mCTSIB would be a useful test to evaluate both residual dizziness and postural imbalance after CRP in BPPV.
-
Keywords: Benign paroxysmal positional vertigo; Dizziness; Posture balance; Surveys and questionnaires
-
중심단어: 양성돌발체위현기증, 어지럼, 자세검사, 설문지
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is common peripheral vestibular disorder [1]. It is caused by dislodged otoconia from the denatured utricular macula displacing into semicircular canals. The most common treatment for BPPV is the canalith repositioning procedure (CRP) specific to each affected canal [2]. Most patients with BPPV make a good recovery and have their vertigo symptoms disappear after successful CRP, but some patients report residual dizziness for a certain period afterward [3]. Most residual dizzinesses are characterized by non-positional, non-rotatory, or persistent imbalance of variable duration.
The cause of residual dizziness after successful CRP remains controversial. According to previous literatures, it is associated with age, high scores on questionnaire with higher self-rated anxiety scores [3], orthostatic hypotension [4], or utricular dysfunction [5]. Thus, residual dizziness after successful CRP seems to be caused by mental state, vestibular insufficiency, or autonomic dysfunction. Alternatively, residual dizziness may be caused by postural instability that remains after CRP. Several studies revealed that long-term disturbance in BPPV patients is linked with vestibule-spinal reflexes by performing static/dynamic posturography [6,7].
The sensory organization test (SOT) of computerized dynamic posturography is useful for evaluating postural instability. It has sub-conditions based on combinations of support surfaces stability, vision availability, and visual surround stationary/moving. However, it is time and cost consuming test for undergoing the process with space requirements. In this respect, Shumway-Cook and Horak [8] proposed an easy and efficient balance test based on SOT, the Clinical Test of Sensory Integration and Balance (CTSIB). It is easily administered with timer and mediumdensity compliant foam. In addition, CTSIB was modified (mCTSIB) because there were no significant differences in scores between conditions with the visual conflict dome and without dome [9]. Park et al. [10] reported that the mCTSIB can be used instead of the SOT in screening to distinguish normality from abnormality in dizzy patients with unilateral vestibulopathy. Both SOT and mCTSIB is a useful test tool for assessing the ability of balance. As mCTSIB has advantage of time and cost-effectiveness, mCTSIB could be used instead of the SOT in the screening test for dizziness. The mCTSIB is now widely used in practice, but few studies have assessed the residual dizziness after CRP in BPPV patients and the results of mCTSIB. The aim of the present study was to investigate the correlation between subjective residual dizziness and objective postural imbalance after successful CRP in BPPV by using questionnaires and mCTSIB.
MATERIALS AND METHODS
We prospectively analyzed patients with BPPV from December 2017 to January 2019. The diagnostic procedure consisted of a detailed clinical history, a neurologic bedside examination, and videonystagmography (VNG). Inclusion criteria included: (1) idiopathic BPPV and (2) confirmed successful CRP (resolution of positional nystagmus and symptoms) on the initial visit day. Since we only included the patients who were successfully treated after just one CRP on initial visit day, it is thought that the possibility of false negatives caused by fatigue of multiple tests was very low. We excluded patients who had the following conditions: (1) a history of inner ear disease, (2) previous surgery or trauma, (3) psychologic or neurologic disorders, (4) failure of successful CRP for any reason, and (5) current use of any medication that affects the central nervous system. All included patients were treated with CRP according to the type of BPPV: posterior semicircular canal, Epley’s maneuver; lateral semicircular canal, Barbeque rotation. In cases of cupulolithiasis, CRP was done after inducing canalolithiasis by head shaking or mastoid vibration.
We used Dizziness Handicap Inventory (DHI), a validated 25-item questionnaire for assessing physical (P) and emotional symptoms (E), and functional impairment (F) for evaluating symptom severity [11]. Also, a visual analog scale (VAS) was utilized with a total of 10 scales ranging from 0 (no dizziness) to 10 (maximum dizziness). Questionnaires of DHI and VAS were conducted initially and at 2 weeks after CRP. All patients were scheduled to return 2 weeks after CRP and resolution of positional nystagmus was confirmed by VNG. All patients were educated to revisit even before 2 weeks when they experience vertigo episodes again. If there were any patients who experienced vertigo episode which suspected recurred BPPV within 2 weeks, they were excluded from the subjects. In addition, if positional nystagmus recurred on follow-up, the patient was excluded from the study. All patients were asked to describe the characteristics of their subjective residual symptoms with DHI and VAS.
The mCTSIB was performed both on the floor and Sunmate medium-density compliant foam (Balance master, Dynamic Systems, San Carlos, CA, USA) with feet together. The subjects are instructed to maintain an upright position during the test for up to 30 seconds. The mCTSIB included four conditions: firm EO, standing on a firm surface with the eyes open; firm EC, standing on a firm surface with the eyes closed; foam EO, standing on a compliant surface with the eyes open; and foam EC, standing on a compliant surface with the eyes closed. Patients have repeatedly examined three trials under the above four conditions. We calculated scores as the average of three trials. The examiner instructed each patient to balance for 30 seconds to assess the center of gravity (COG). Equilibrium scores and normal or abnormal findings were determined according to the manufacturer’s criteria for each subject, considering age and height [12]. For each condition, we recorded the mean COG sway velocity. Briefly, an objective measurement of body sway is obtained using a computer-controlled platform that monitors body sway by three pressure-sensing strain gauges located on the vertices of an equilateral triangle drawn on the platform [13].
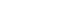
We divided patients into two groups according to VAS at 2 weeks after CRP; the non-RD group (no residual dizziness) and the RD group (residual dizziness). We defined the non-RD group as patients with zero VAS at 2 weeks after CRP. Otherwise, if VAS at 2 weeks after CRP was more than one, we defined the patient as the RD group. The following data were compared between non-RD and RD groups; age, sex, affected semicircular canal, number of CRP, DHI score, and mCTSIB parameters. Also, we evaluated the correlation between DHI score and mCTSIB parameters. Fig. 1 shows the schematic study protocol. The study protocol was approved by the Institutional Review Board at Kangbuk Samsung Hospital with a waiver for informed consent (No. KBSMC2019-10-005).
Statistical analysis was performed with Mann-Whitney test, chi-square test, and Pearson correlation coefficient using IBM SPSS Statistics for Windows ver. 24.0 (IBM Corp., Armonk, NY, USA), and p-values below 0.05 were considered statistically significant.
RESULTS
Table 1 shows the demographics and clinical characteristics of the study population. Five patients demonstrated recurrent positional nystagmus at follow-up, they were excluded from the study. Finally, 31 patients were enrolled and the mean age of them was 51.73±10.12 years with 13 males and 18 females. Eighteen patients (58.1%) reported residual dizziness at followup after the successful CRP, even though positional nystagmus was negative (RD group). Age, sex, and side of BPPV did not show significant differences between RD and non-RD groups.
In the analysis of symptom characteristics in the RD group, the most common residual symptom was lightheadedness, which is a kind of feeling of dizziness as if you might faint or be close to passing out (n=10). The second most common symptom was a floating sensation, which is a feeling of imbalance as if you might walk on the cloud (n=8). Horizontal semicircular canal BPPV constituted the largest proportion of cases (55.5%) in the RD group. There were no differences in RD and non-RD groups with respect to involved semicircular canals and the number of CRP (Table 2).
Both groups showed a significant decrease in total post-DHI score after successful CRP and subjects of the RD group showed significantly higher post-DHI score than the non-RD group. The post-DHI(E) score was the main component of the total post-DHI score in the RD group (mean, 9.81±4.63). Also, the difference between pre-DHI(E) and post-DHI(E) scores was less than other subscales including (P) and (F) in the RD group (Table 3). In the non-RD group, five subjects showed post-DHI more than zero. They also had a high component of (E) in post-DHI.
Subjects of the RD group showed a significantly high number of abnormal mCTSIB than the non-RD group (RD group, 1.28±1.13; non-RD group, 0.31±0.48; p<0.05) (Table 4). Furthermore, the mean COG sway velocity (°/sec) was 1.18 in the RD group and 0.89 in the non-RD group respectively. There was a significant difference between RD and non-RD groups.
In addition, the post-DHI score and the number of abnormal mCTSIB showed a statistically significant correlation in the RD group (p<0.05) (Fig. 2). However, there was no significant correlation between the post-DHI score and mean COG sway velocity of mCTSIB in the RD group (Table 5).
DISCUSSION
The results of this study showed several findings similar to other studies. First, residual dizziness was relatively common (58.1%) in the follow-up period. The overall prevalence of residual dizziness after successful CRP in BPPV patients is ranged from 31% to 61% according to other literatures [14]. Second, residual dizziness is often described as a lightheadedness or floating sensation in absence of vertigo, or short-lasting unsteadiness occurring during head movements, standing, or walking and the lightheadedness was most common in our study (55.6%). Moreover, residual dizziness seems not to be related with involved canal, sex, and the number of CRP as described in previous reports [4,15]. Our study also demonstrated that age, sex, side, involved canal, and the number of CRP did not show significant differences between RD and non-RD groups.
Although there have been a lot of findings in literatures about residual dizziness after successful CRP in BPPV patients, possible causes are still under debate. The possible explanations include the persistence of debris in the canal insufficient to provoke noticeable positional nystagmus, a utricular dysfunction, a coexisting vestibular disease, or an incomplete central adaptation. However, these hypotheses have not yet been supported by definitive data.
Standing balance is a complex process that depends solely on the integration of the visual, vestibular, somatosensory systems, central coordination, and muscular adjustment [16]. If any of them has an impairment, a patient could feel an imbalance in standing position. Furthermore, there are some evidences that standing imbalance after successful CRP could be produced by a little amount of residual debris that does not generate positional nystagmus, a coexisting vestibular disease, an incomplete central adaptation or persistent postural-perceptual dizziness [3,17]. In the present study, we aimed to evaluate the correlation between subjective residual dizziness and somatosensory system.
Although the mCTSIB does not specify the exact nature of a patient’s balance problem, it is useful in differentiating between individuals with and without vestibular disorders. It is also helpful for obtaining data about patients’ performance in daily life [9]. It contains four conditions including firm EO, firm EC, foam EO, and foam EC. Patients underwent three test repetitions under each of the four conditions described above, and scores were calculated as the average of three trials. Investigating the somatosensory system in this way may give more insight into the pathogenesis of residual dizziness in BPPV. Our study investigated the integrated controls of the vestibulospinal reflex needed to maintain the standing position. The mCTSIB which involves the analysis of body sway during standing is a quite reliable means of studying the vestibulospinal reflex.
In this study, subjects of the non-RD group maintained an upright position during the test up to 30 seconds in each condition significantly better than subjects of the RD group. Also, many subjects in the RD group could not maintain position in the condition of foam EC, suggesting a prevalence of visual cues in balance control. Visual dependence implies subjects who preferentially use vision, as opposed to vestibular or proprioceptive input, for spatial orientation and postural control [18]. An increased visual dependence has been demonstrated in patients after an acute vestibular disorder [19] and posturography in the condition of foam EC is thought to be more specific in evaluating visual dependence. We assumed that the standing imbalance of the patients in the RD group could be due to some acute sensory conflict between the affected vestibular system and vision. In addition, these results support that standing balance during normal physical activity can be one of the causes of residual dizziness in BPPV patients.
Several dizziness questionnaires have been used to quantify residual symptoms after CRP in BPPV patients. In the present study, we used the DHI introduced by Jacobson and Newman in 1990 [11]. The DHI is a validated scale of impairment widely used in clinical practice and many clinical studies. A high DHI score is associated with an increased level of handicap. In the present study, subjects of the RD group showed significantly higher post-DHI score than non-RD group. Interestingly, the post-DHI(E) score was the main component of total post-DHI score in the RD group (mean, 9.81±4.63). Also, the difference between pre-DHI(E) and post-DHI(E) scores was less than other subscales including (P) and (F) in the RD group. These results are in agreement with previous studies. Mendel et al. [20] reported that residual feelings of anxiety and depression persisted in patients suffering from peripheral vestibular disorders. This would be related to a great anxiety level due to the intrinsic unpredictability of the BPPV itself even after successful CRP [14]. Furthermore, BPPV patients who suffer from anxiety disorders show longer-lasting and more disabling dizziness after the resolution of acute vertigo. Anxiety plays an additional role in dizziness and can be considered in some situations as a somatoform disorder caused by stressful events. Thus, we should pay attention to emotional component in follow-up period for BPPV.
Previous studies have attempted to determine the correlation between the severity of subjective dizziness and objective measurements of balance performance. In general, quantitative measurements of the patients’ performance did not necessarily correlate with self-perceived dizziness handicaps because of the difference in several clinical factors. Even though mCTSIB assesses somatosensory system, rather than vestibulo-ocular reflex, our study showed relatively well correlation between postural sway and DHI scale. We can postulate that a vestibular dysfunction after BPPV including persistence of a little debris in the canal or utricular dysfunction may lead to a proprioceptivelike disturbance, which in turn, temporarily alters vestibulospinal reflexes, finally resulting in standing imbalance. Stambolieva and Angov [21] suggested that the physical treatment of BPPV is not able to treat vestibular system which has already been damaged by the otoconia. Thus, the postural disturbance might be due to the presence of otoconia, which modifies the dynamics of the affected semicircular canal and changes the sensibility of the motion-sensing receptors. From the physiological point of view, the persistence of debris in the semicircular canal can alter the tonic discharge from the affected labyrinth. Such functional asymmetry can induce a new adaptation, through a rebalancing of the activity between the vestibular nuclei [15]. This new condition tends to neutralize the imbalance produced in the peripheral vestibular system. Also, post-DHI(E) score was main component of total post-DHI score in the RD group. Possibly, overlapping neural circuits between anxiety and the balance control system may provoke increased anxiety levels in patients after BPPV, and patients developing higher anxiety have an incomplete central adaptation [22].
There are some limitations of this study. First, the duration of vertigo was not considered in the present study. Because the longer otoconia remain floating in the endolymph before treatment, the longer time for recovery and central adaptation would be needed. So, time period before diagnosis and treatment should be checked for evaluating residual dizziness in BPPV. Second, we did not utilize ocular vestibular-evoked myogenic potentials (oVEMPs) for patients with BPPV. There have been several studies that patients with BPPV have abnormal utricular function, and this dysfunction remains even after a successful CRP. Accordingly, abnormal oVEMPs results can be observed in patients with residual dizziness. But oVEMPs could not show high test-retest reliability clinically. Also, there have been controversies that oVEMPs reflect utricular dysfunction perfectly. Therefore, various vestibular function tests including subjective visual vertical or oVEMPs would be needed in the future study.
We accessed residual dizziness of patients with BPPV after successful CRP using DHI and compared these findings using mCTSIB in RD and non-RD groups. We demonstrated the correlation between DHI score and mCTSIB in patients with residual dizziness. Post-DHI(E) scores in the RD group were high enough to show that emotion and postural imbalance might have deeply involved each other. Therefore, mCTSIB would be a useful test to evaluate both residual dizziness and postural imbalance after CRP in BPPV. However, additional pathophysiological evidence is required for postural imbalance in order to confirm our results.
ARTICLE INFORMATION
-
No potential conflict of interest relevant to this article was reported.
Fig. 1.Schematic of the study protocol. All included subjects were conducted canalith repositioning procedure (CRP) for the benign paroxysmal positional vertigo (BPPV) at initial. Questionnaires of Dizziness Handicap Inventory and visual analog scale (VAS) were conducted initially and at 2 weeks after CRP. The modified Clinical Test of Sensory Integration and Balance (mCTSIB) was also performed 2 weeks after CRP. We divided patients into two groups according to VAS at 2 weeks after CRP; residual dizziness (RD) group and non-RD group. FU, follow-up.
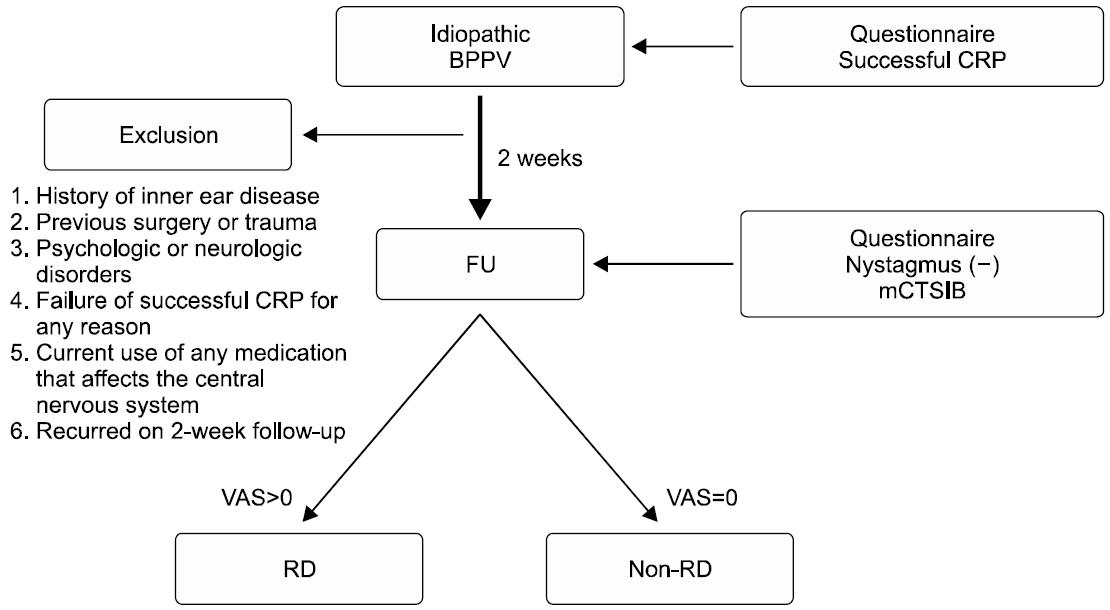
Fig. 2.Correlation between the number of abnormal modified Clinical Test of Sensory Integration and Balance (mCTSIB) and postDizziness Handicap Inventory (DHI) score in residual dizziness group. They showed a statistically significant correlation (*p<0.05, r=–0.809).

Table 1.Demographics of the study population
|
Variable |
RD group (n=18) |
non-RD group (n=13) |
p-valuea)
|
|
Mean age (yr) |
53.4 |
51.1 |
0.51 |
|
Sex |
|
|
|
|
Male |
8 |
5 |
0.56 |
|
Female |
10 |
8 |
|
|
Side, right/left |
7/11 |
6/7 |
0.74 |
Table 2.Group evaluation according to type of benign paroxysmal positional vertigo and number of CRP
|
Variable |
RD group |
Non-RD group |
p-valuea)
|
|
Involved canal |
|
|
|
|
Posterior |
5 |
5 |
0.47 |
|
Horizontal |
10 |
8 |
|
|
Anterior |
1 |
0 |
|
|
Multiple |
2 |
0 |
|
|
No. of CRPs |
|
|
0.59 |
|
1 |
14 |
10 |
|
|
>1 |
4 |
3 |
|
Table 3.Analysis of DHI scores between groups
|
Variable |
DHI score
|
|
RD group |
non-RD group |
p-value |
|
Pre-VAS |
8.22±1.81 |
8.87±2.02 |
0.49 |
|
Post-VAS |
2.82±1.43 |
0 |
<0.05a)
|
|
Pre-DHI |
50.82±24.38 |
46.12±21.92 |
0.32 |
|
Post-DHI |
19.22±21.12 |
2.69±4.30 |
<0.05a)
|
|
Post-DHI(E) |
9.81±4.63 |
1.16±0.98 |
<0.05a)
|
|
Post-DHI(F) |
5.77±3.19 |
1.05±0.81 |
<0.05a)
|
|
Post-DHI(P) |
3.06±1.23 |
0.7±0.33 |
<0.05a)
|
|
Δ DHI(E) |
5.67±2.55 |
15.29±8.17 |
<0.05a)
|
|
Δ DHI(F) |
12.41±6.67 |
14.22±7.81 |
0.45 |
|
Δ DHI(P) |
10.95±5.11 |
11.70±4.24 |
0.40 |
Table 4.Number of abnormal mCTSIB in each condition and mean COG sway velocity
|
Variable |
RD group |
Non-RD group |
p-value |
|
No. of abnormal mCTSIB |
|
|
|
|
Firm EO |
1 |
0 |
|
|
Firm EC |
4 |
0 |
|
|
Foam EO |
10 |
2 |
|
|
Foam EC |
8 |
1 |
|
|
Mean COG sway velocity (°/sec)a)
|
1.18 |
0.89 |
0.004 |
Table 5.Correlation between DHI score and mean COG sway velocity of mCTSIB in the RD group
|
Condition |
Coefficient of correlation (r) |
p-valuea)
|
|
Firm EO |
0.053 |
0.68 |
|
Firm EC |
‒0.145 |
0.20 |
|
Foam EO |
0.080 |
0.73 |
|
Foam EC |
‒0.259 |
0.29 |
REFERENCES
- 1. von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry 2007;78:710–5.ArticlePubMed
- 2. Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg 2017;156(3_suppl):S1–S47.Article
- 3. Martellucci S, Pagliuca G, de Vincentiis M, Greco A, De Virgilio A, Nobili Benedetti FM, et al. Features of residual dizziness after canalith repositioning procedures for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 2016;154:693–701.ArticlePubMed
- 4. Kim HA, Lee H. Autonomic dysfunction as a possible cause of residual dizziness after successful treatment in benign paroxysmal positional vertigo. Clin Neurophysiol 2014;125:608–14.ArticlePubMed
- 5. Seo T, Shiraishi K, Kobayashi T, Mutsukazu K, Fujita T, Saito K, et al. Residual dizziness after successful treatment of idiopathic benign paroxysmal positional vertigo originates from persistent utricular dysfunction. Acta Otolaryngol 2017;137:1149–52.ArticlePubMed
- 6. Giacomini PG, Alessandrini M, Magrini A. Long-term postural abnormalities in benign paroxysmal positional vertigo. ORL J Otorhinolaryngol Relat Spec 2002;64:237–41.ArticlePubMed
- 7. Teggi R, Quaglieri S, Gatti O, Benazzo M, Bussi M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo. ORL J Otorhinolaryngol Relat Spec 2013;75:74–81.ArticlePubMed
- 8. Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance: suggestion from the field. Phys Ther 1986;66:1548–50.PubMed
- 9. Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther 1993;73:346–54.ArticlePubMed
- 10. Park MK, Kim KM, Jung J, Lee N, Hwang SJ, Chae SW. Evaluation of uncompensated unilateral vestibulopathy using the modified clinical test for sensory interaction and balance. Otol Neurotol 2013;34:292–6.ArticlePubMed
- 11. Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg 1990;116:424–7.ArticlePubMed
- 12. N atus Medical Inc. NeuroCom SMART Balance Master [Internet]. San Carlos, CA, Natus Medical Inc. 2021 [cited 2021 May 21]. Available from: https://cordialmedical.nl/public/products/015367A_SMART-BM_EN-US_lores.pdf.
- 13. Freeman L, Gera G, Horak FB, Blackinton MT, Besch M, King L. Instrumented test of sensory integration for balance: a validation study. J Geriatr Phys Ther 2018;41:77–84.ArticlePubMedPMC
- 14. Giommetti G, Lapenna R, Panichi R, Mobaraki PD, Longari F, Ricci G, et al. Residual dizziness after successful repositioning maneuver for idiopathic benign paroxysmal positional vertigo: a review. Audiol Res 2017;7:178. ArticlePubMedPMC
- 15. Seok JI, Lee HM, Yoo JH, Lee DK. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J Clin Neurol 2008;4:107–10.ArticlePubMedPMC
- 16. Oliva Domínguez M, Bartual Magro J, Dañino González JL, Dañino González G, Roquette Gaona J, Bartual Pastor J. Postural control according to the age in patients with benign paroxysmal positional vertigo. Rev Laryngol Otol Rhinol (Bord) 2005;126:267–70.PubMed
- 17. Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, Bronstein A. Diagnostic criteria for persistent postural- perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res 2017;27:191–208.ArticlePubMedPMC
- 18. Agarwal K, Bronstein AM, Faldon ME, Mandalà M, Murray K, Silove Y. Visual dependence and BPPV. J Neurol 2012;259:1117–24.ArticlePubMed
- 19. Nagarkar AN, Gupta AK, Mann SB. Psychological findings in benign paroxysmal positional vertigo and psychogenic vertigo. J Otolaryngol 2000;29:154–8.PubMed
- 20. Mendel B, Bergenius J, Langius A. Dizziness symptom severity and impact on daily living as perceived by patients suffering from peripheral vestibular disorder. Clin Otolaryngol Allied Sci 1999;24:286–93.ArticlePubMed
- 21. Stambolieva K, Angov G. Postural stability in patients with different durations of benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol 2006;263:118–22.ArticlePubMed
- 22. Wei W, Sayyid ZN, Ma X, Wang T, Dong Y. Presence of anxiety and depression symptoms affects the first time treatment efficacy and recurrence of benign paroxysmal positional vertigo. Front Neurol 2018;9:178. ArticlePubMedPMC
Citations
Citations to this article as recorded by

- Immediate Improvement in Subjective Visual Vertical and Disequilibrium Predicts Resolution of Benign Paroxysmal Positional Vertigo Following Single Canalith Repositioning Maneuver
Christine C. Little, Zachary G. Schwam, Marc Campo, James Gurley, Bryan Hujsak, Maura K. Cosetti, Jennifer Kelly
Otology & Neurotology Open.2022; 2(3): e014. CrossRef - Gait and Postural Control Characteristics among Individuals with Benign Paroxysmal Positional Vertigo: A Scoping Review
Haziqah Nasruddin, Maria Justine, Haidzir Manaf
Malaysian Journal of Medicine and Health Sciences.2022; 18(s15): 377. CrossRef






 KBS
KBS
 PubReader
PubReader ePub Link
ePub Link Cite
Cite