글로포시네이트암모늄 중독에서 관찰된 신경이안과 소견
Neurotological Findings in a Patient with Glufosinate Ammonium Intoxication
Article information
Abstract
Abstracts
The upward deviation could be explained by loss of inhibitory inputs from the cerebellum onto the brainstem anterior semicircular canal projections for upward vestibulo-ocular reflex, which would lead to an upward bias in static eye position. Therefore, upward gaze deviation has been reported in comatose patients after resuscitation and diffuse cerebrocerebellar damage sparing the brainstem. Herein, we report a patient with ingestion of glufosinate ammonium presented with cerebellar ataxia and ocular motor findings suggestive of cerebellum involvement such as upward gaze tendency, spontaneous downbeat, gaze-evoked nystagmus, perverted head impulse test, and impaired smooth pursuit.
INTRODUCTION
Glufosinate ammonium (GLA), a nonselective herbicide, is made from a phosphinic acid derivative of glutamate. It is an ammonium salt of phosphinothricin (D, L-homoalanine-4-[methyl] phosphinate) that is structurally related to glutamate. It acts by inhibiting the synthesis of glutamine in plants and mammals [1]. Various neurological symptoms including loss of consciousness, convulsion, and memory impairment due to acute GLA intoxication have been reported [1–7]. However, no study has reported brainstem and cerebellar dysfunction associated with GLA. Here we report a patient with severe imbalance and ocular motor findings following ingestion of a GLA herbicide in an attempted suicide. This study was approved by the Institutional Review Board of Chungnam National University Hospital (CNUH 2020-10-046).
CASE REPORT
A 68-years-old woman was presented in emergency room due to GLA intoxication with ingestion of about 50 mL of BASTA. Her consciousness was alert. She was well-cooperative when arriving at the hospital. She underwent nasogastric irri-gation and charcoal lavage after exposure. She was admitted to Department of Emergency Medicine for the correction of hypoosmolar hyponatremia. Her serum Na level was 127.9 mEq/L (normal range, 135–150 mEq/L). Her serum osmolality was 269 mOsmol/L (normal range, 275–295 mOsmol/L). She received supportive care. Twelve hours after ingestion of GLA, she complained of oscillopsia, dizziness, and imbalance. Motor and sensory abnormalities were not observed. Saccade was hypometric and gain of smooth pursuit was decreased bi-laterally. She showed upward gaze deviation tendency with ocular bobbing and downbeat nystagmus (Fig. 1A, B; Supplementary Video 1). Horizontal nystagmus was noted in either horizontal gaze (Fig. 1C). Her spontaneous downbeat became more prominent in straight head hanging and Dix-Hallpike maneuver. The horizontal head impulse showed upward trajec-tory, i.e., perverted and cross-coupled response (Fig. 1A). Limb ataxia was shown in cerebellar function test. She could not walk independently. Her spontaneous nystagmus during sinusoidal rotation with fixation stimuli was not suppressed in rotary chair test. The gain of vestibulo-ocular reflex (VOR) during rotary chair test was distributed in a range of 0.25 to 0.65, which was increased mildly during visually enhanced VOR. Time constants of step velocity were within normal limit (average: rightward, 8.82 seconds; leftward, 9.59 seconds). Her tilt suppression was preserved (right tilt suppression index, 48.12%; left tilt suppression index, 50.42%). The responses to bithermal caloric stimuli were preserved in both ears (slow peak velocities in the right ear cool:in the left ear warm:in the right ear warm:in left the ear cool = 19:22:14:27 [°/second], caloric paresis = 24%). Brain magnetic resonance imaging and cerebrospinal fluid study were unremarkable. On the 2nd hospital day, her dizziness and imbalance improved with disappearance of upward gaze deviation. Her spontaneous downbeat nystagmus disappeared on the 3rd day. She was discharged from the hospital with recovery to previously healthy state on the 5th day of hospitalization.
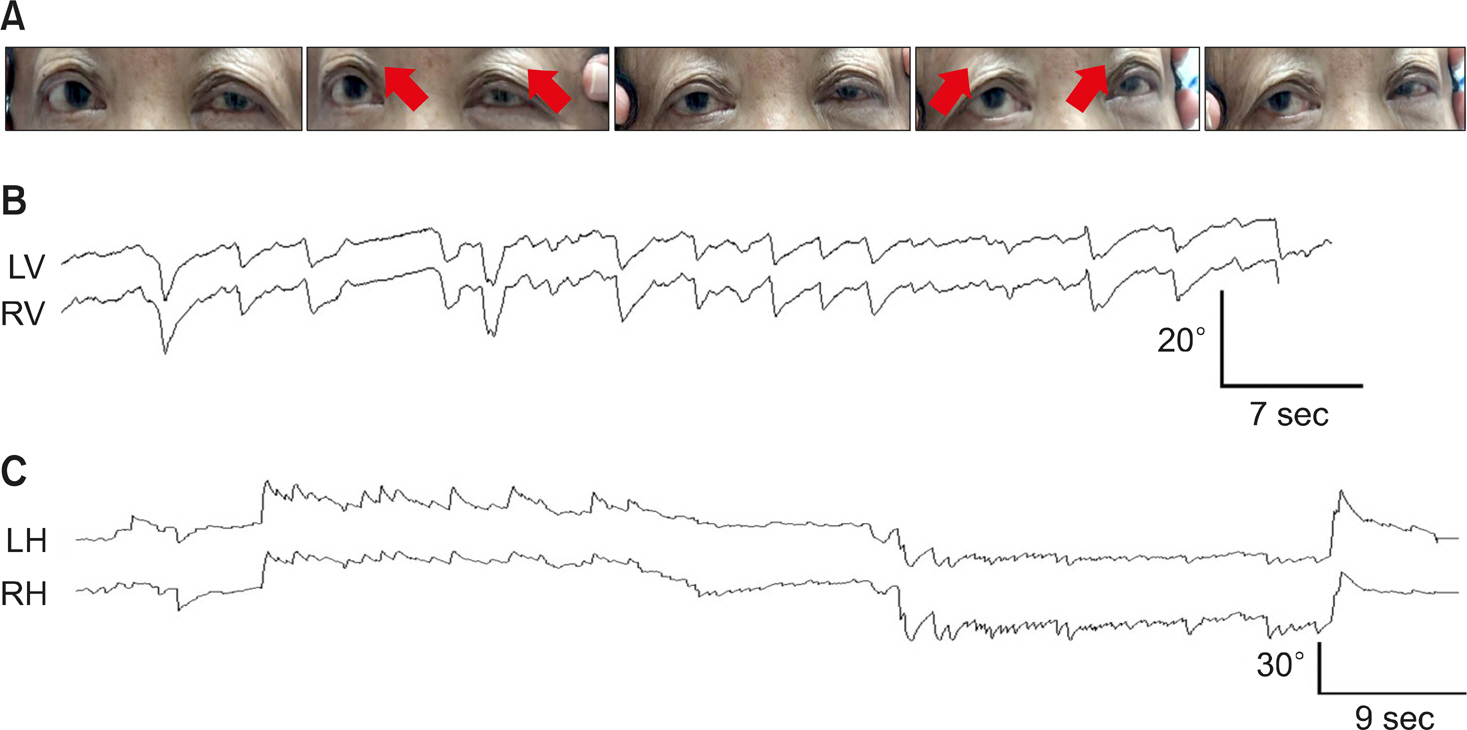
(A) The horizontal head impulse shows upward trajectory. (B) The patient shows ocular bobbing and spontaneous downbeat nystagmus. Upward deflection indicates upward (from the patient's perspective) eye motion. (C) Oculography shows right beat nystagmus in rightward gaze and left beat nystagmus in leftward gaze. Upward deflection indicates rightward (from the patient's perspective) eye motion. LV, vertical position of the left eye; RV, vertical position of the right eye; LH, horizontal position of the left eye; RH, horizontal position of the right eye.
DISCUSSION
Our patient with ingestion of GLA presented with cerebellar ataxia and ocular motor findings suggestive of cerebellum involvement such as upward gaze tendency, spontaneous down-beat, gaze-evoked nystagmus, perverted head impulse test, and impaired smooth pursuit. Most neurological manifestations related to GLA intoxication were found to be mental change, seizure, and memory dysfunction [1,3–5]. We could find one report on neurotological finding related to GLA. It reported bilateral 6th nerve palsy and nystagmus [6]. Detailed neurotological finding including the pattern of nystagmus has not been reported. To the best of our knowledge, this is the first report of brainstem and cerebellum dysfunction related to GLA intoxication with a maintenance of consciousness. How can we explain the findings in our patient? Although the exact mechanism of GLA intoxication is unknown in human, GLA is structurally similar to glutamate, a neurotransmitter that interferes with its proper function [8]. In view of the fact that both mossy fibers and granule cells (and thus the overwhelming majority of synapses in the cerebellum) are glutamatergic, it is not surprising that the administration of glutamate antagonists can cause a marked worsening of cerebellar function in patients with cerebellar lesions [9]. Especially, the vestibulocerebellum consisting mainly of flocculus and nodulus receives signals through the mossy fiber and projects back to vestibular nuclei [9,10]. Damage to this region can cause disturbances of locomotion and equilibrium with various neuro-ophthalmologic findings as shown in our patient. Clinical findings of bilateral lesions affecting the cerebellar flocculus and paraflocculus are as follows; impaired VOR fixation, impaired ability to suppress caloric vestibular nystagmus by fixating on a stationary target, impaired gaze-holding function, downbeat nystagmus, and abnormal head impulse [10]. The present case suggests the GLA can induce brainstem and cerebellar dysfunction in human.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Supplementary material can be found via (https://doi.org/10.21790/rvs.2020.19.4.138).
rvs-19-4-138-suppl1.mp4