Abstract
- The anterior cerebellar vermis has been known to act in coordination of gait and postural adjustment of the trunk and legs. However, oculomotor abnormalities in an isolated anterior vermian lesion have not been described in the literature. A 59-year-old man presented with acute non-rotatory dizziness and disequilibrium. Neuro-ophthalmologic examination found impaired smooth pursuit and hypometric saccades in the contralesional direction, and disconjugate ipsiversive ocular torsion, but without spontaneous or gaze-evoked nystagmus. Imaging study showed an infarction restricted to the rostral end of right cerebellar vermis involving the lingual and central lobules. The anterior cerebellar vermis participates in the maintenance of axial posture and gait, and also in the control of ocular motor and vestibular systems.
-
Keywords: Cerebellum; Cerebellar vermis; Gait; Smooth pursuit; Saccades; Ocular torsion
-
중심단어: 소뇌, 소뇌충부, 보행, 원활추종안구운동, 신속안구운동, 안구회선
INTRODUCTION
The anterior cerebellar vermis, consisting of the lingula, central lobule, and culmen, has been known to participate in coordination of gait and postural adjustments of the trunk and legs. Since superior cerebellar artery (SCA) infarctions commonly occur in the territory of the lateral branch, and frequently accompany other infarctions in the territory of inferior cerebellar or rostral basilar arteries, only a few studies have described the clinical features in lesions restricted to the anterior vermis that is usually supplied by the medial branch of the SCA (mSCA) [1]. Isolated infarctions in the territory of mSCA have produced dysarthria, prominent gait ataxia with axial lateropulsion, mild appendicular ataxia, and extensor posturing of the neck, trunk, and extremities [1,2]. Injury of the midline anterior vermian neurons causes loss of inhibitory projections upon the ipsilateral vestibulospinal tract neurons within the lateral vestibular nucleus, and subsequent disinhibition of the extensor muscle tone, leading to axial lateropulsion [2]. The dorsal spinocerebellar fibers carrying the unconscious proprioceptive information from the leg and lower trunk end in the lingula, which is associated with control of the gait and posture [3]. While unsteadiness in the stance and gait is well known in anterior cerebellar strokes, oculomotor abnormalities in an isolated anterior vermian lesion have not been delineated in the literature. Herein, we report a patient with an isolated rostral vermian infarction who showed impaired smooth pursuit and disconjugate ocular torsion.
CASE REPORT
A 59-year-old man with hypertension, diabetes mellitus, and coronary artery disease suddenly developed non-rotatory dizziness and disequilibrium while walking up the stairs. He felt heaviness in both legs and unsteadiness during stance and gait. He did not have nausea, vomiting, headache, diplopia, dysarthria, or other neurologic symptoms. One day later, neurologic examination could not find abnormal head and neck posture, skew deviation, spontaneous nystagmus with and without visual fixation using Frenzel goggles, or gaze-evoked nystagmus. Bedside head impulse tests were normal. He could stand unaided, but showed a sway from side to side on natural and tandem gaits and during Romberg testing with the feet apposed. There was no dysarthria, tremor, or gaze palsy. The remainder of neurologic examination was normal including vibration, proprioception, past pointing, muscle tone, and deep tendon reflexes.
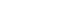
Video-oculography documented no spontaneous or gaze-evoked nystagmus. Leftward smooth pursuit was impaired in response to sinusoidal target motion at a peak velocity of 10º/sec (gain at 0.36, age-matched normal±1standard deviation>0.71±0.11) whereas rightward smooth pursuit was preserved (gain at 0.51, right-left asymmetry at 16.79%) (Figure 1A). Ipsilesional saccades were normal, but contralesional saccades were mildly hypometric with a mean gain at 84.4% (Figure 1B). Head turning to either side while supine produced small apogeotropic nystagmus with a slow-phase velocity at 3.5º/sec for about 10 seconds but without vertigo. Video head impulse test documented normal vestibulo-ocular reflex gains for horizontal and vertical canals on both sides. He also showed normal gains and phases for the vestibulo-ocular reflex during sinusoidal harmonic acceleration and normal time constants of the perand post-rotatory nystagmus during step velocity rotations. Tilt suppression of the post-rotatory nystagmus was normal. Bithermal caloric tests were normal. Fundus photographs showed abnormal extortion of the right eye (15.1º, normal range of 0 to 12.6º), but normal torsional position of the left eye (9.1º) (Figure 1C). There was no abnormal tilt of the subjective visual vertical. Cervical and ocular vestibular evoked myogenic potential tests were normal. Diffusion-weighted magnetic resonance imaging (MRI) showed an infarction restricted to the right lingula and central lobule (Figure 1D–E). Cerebral angiography showed no stenoocclusive lesion. The dizziness and gait unsteadiness improved over the following five days, and eight months later oculography showed normal smooth pursuit in both directions (rightward gain at 0.62, and leftward gain at 0.61, right-left asymmetry at 1.31%).
DISCUSSION
Our patient with a unilateral infarction restricted to the anterior midline vermis presented with acute non-rotatory dizziness and unsteadiness during gait, and neuro-ophthalmologic evaluation showed impaired smooth pursuit and hypometric saccades in the contralesional direction, and disconjugate ocular torsion. This suggests that anterior vermis contributes to ocular motor control as well as posture and gait.
Multiple areas of the cerebral cortex, brainstem and cerebellum are involved in the generation and coordination of smooth pursuit eye movements. Neuronal signals for smooth pursuit eye movements are initiated in the frontal and parietal eye centers, and then mediated by the pontine nuclei [4]. The pontine nuclei project to the vestibulocerebellum and the cerebellar oculomotor vermis that includes the declive, folium, and tuber (Figure 1F) [4]. The uvula and part of the pyramid are also commonly damaged in patients with deficient gain of the horizontal sinusoidal smooth pursuit eye movements [5]. The smooth pursuit gain was decreased bilaterally, but significantly so for ipsilateral movements in posterior vermian lesions [6]. Unilateral stroke involving the flocculus and the paraflocculus, corresponding to the tonsil in humans, also produced impaired horizontal smooth pursuit to the lesion side [7]. Our patient shows that the anterior cerebellar vermis may play a role in the smooth pursuit circuitry mainly in the contralateral direction. Of note, unilateral inactivation of the fastigial nucleus decreases the gain of contralateral smooth pursuit [8]. Since many tracts pass and end in the rostral vermis, it is difficult to draw a convincing conclusion on the neuronal structure responsible for contralesionally impaired smooth pursuit in our patient with isolated rostral vermian infarction. Given that the fastigial oculomotor region receives inputs from the Purkinje cells of the dorsal vermis and also axon collaterals from pontine nuclei [9], however, part of the efferent fibers from the fastigial nucleus are most likely to be injured while passing through the rostral vermis.
In our patient, saccades were largely preserved except mild hypometria to the intact side. Hypermetria of centripetal saccades has been reported in monkeys after damage to either the posterior vermis and underlying fastigial nuclei or the fastigial nuclei only [6,10]. Experimental lesions restricted the oculomotor vermis in primates, with sparing of the deep cerebellar nuclei, also shows impairments of every aspect of saccades: latency, amplitudes, velocity, acceleration, and adaptation [11]. Similarly, patients with cerebellar lesions affecting the posterior vermis show impairments of both smooth pursuit and saccades [6]. Hypometric bias of contralesional saccades in our patient with unilateral rostral vermian lesion may be ascribed to partial injury of the fastigial nucleus outputs in the rostral cerebellum, as inferred above, before decussation in the superior cerebellar peduncle. The fastigial nucleus normally facilitates contralateral saccades. Of note, in contrast to our case, a patient with unilateral rostral cerebellar infarction showed saccadic contrapulsion, contralesional hypermetria and ipsilesional hypometria that were attributed to damage to the cerebellar cortex and the deep nuclei, or their projections in the superior cerebellar peduncle [12]. Further oculographic evaluation with well-demarcated unilateral lesions restricted to the rostral cerebellum will confirm the unidirectional saccadic bias found in our patient.
The ocular tilt reaction (OTR), consisting of the triad of head tilt, ocular torsion and skew deviation, indicates a vestibular tone imbalance in the roll plane. In patients with cerebellar nodular lesions, loss of inhibition leads to an increase in tonic resting activity of the otolith neurons in the ipsilateral vestibular nucleus, and consequently contraversive conjugate ocular torsion [13]. Contraversive OTR is also associated with lesions involving the cerebellar dentate nucleus [14]. Deep cerebellar nuclei including the fastigial and dentate nuclei are connected to the vestibular nuclei, and neurons of these cerebellar nuclei respond to head and body tilts along the longitudinal axes [14]. In addition to the graviceptive pathways from the utricle, the OTR can be elicited from the vertical semicircular canal inputs. In a medullary lesion involving the ascending pathway from the posterior canal, the OTR is characterized by disconjugate ocular torsion with an excyclotorsion of the ipsilateral eye [15]. Our patient showed ocular extorsion only in the ipsilesional eye, but without other components of OTR, which implies that the anterior vermis may have a role in modulating the vestibular signals from the ipsilateral posterior canal.
In summary, we report impaired smooth pursuit and hypometric saccades in the contralesional direction, and disconjugate ipsiversive ocular torsion in a patient with an isolated unilateral anterior vermian infarction that produced dizziness and gait unsteadiness. The anterior cerebellar vermis mainly participates in the maintenance of axial posture and gait, but also in the control of ocular motor and vestibular systems. The presence of normal horizontal head impulse tests, direction-changing nystagmus in eccentric gaze, or skew deviation is widely accepted as sensitive for stroke than early MRI in acute vestibular syndrome. Furthermore, our patient shows the importance of acute gait unsteadiness, asymmetrically impaired smooth pursuit and saccadic dysmetria, and disconjugate ocular torsion as the manifestation of vertebrobasilar ischemic stroke.
ARTICLE INFORMATION
-
No potential conflict of interest relevant to this article was reported.
Figure 1.(A) While tracking a sinusoidal target moving at a peak velocity of 10°/sec, the smooth pursuit gain, the ratio of peak eye velocity to peak stimulus velocity, was decreased to the left (0.36, age-matched normal±1SD>0.71±0.11) whereas that was preserved to the right (0.51). (B) Ipsilesional saccades were normal, but contralesional saccades are mildly hypometric with a mean gain at 84.4%. (C) Fundus photographs show abnormal extortion of the right eye (15.1°, normal range=0°–12.6°) but normal torsional position in the left eye. (D) Diffusion-weighted imaging shows an infarction involving the rostral end of right cerebellar vermis, including the lingual and central lobules (E). An illustrative drawing (F) of the fastigial nucleus, the cerebellar vermis, and the ischemic lesion (black). SD, standard deviation.
REFERENCES
- 1. Sohn SI, Lee H, Lee SR, Baloh RW. Cerebellar infarction in the territory of the medial branch of the superior cerebellar artery. Neurology 2006;66:115–7.ArticlePubMed
- 2. Ringel R, Culberson J. Extensor tone disinhibition from an infarction within the midline anterior cerebellar lobe. J Neurol Neurosurg Psychiatry 1988;51:1597–9.ArticlePubMedPMC
- 3. Lee H. Isolated body lateropulsion caused by a lesion of the rostral vermis. J Neurol Sci 2006;249:172–4.ArticlePubMed
- 4. Leigh RJ, Zee DS. The neurology of eye movements. 5th ed. New York: Oxford Univ Press; 2015. p.289–59.
- 5. Baier B, Stoeter P, Dieterich M. Anatomical correlates of ocular motor deficits in cerebellar lesions. Brain 2009;132:2114–24.ArticlePubMed
- 6. Vahedi K, Rivaud S, Amarenco P, Pierrot-D eseilligny C. Horizontal eye movement disorders after posterior vermis infarctions. J Neurol Neurosurg Psychiatry 1995;58:91–4.ArticlePubMedPMC
- 7. Zee DS, Yamazaki A, Butler PH, Gucer G. Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 1981;46:878–99.ArticlePubMed
- 8. Joshi AC, Das VE. Muscimol inactivation of caudal fastigial nucleus and posterior interposed nucleus in monkeys with strabismus. J Neurophysiol 2013;110:1882–91.ArticlePubMedPMC
- 9. Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol 1990;302:330–48.ArticlePubMed
- 10. Vilis T, Hore J. Characteristics of saccadic dysmetria in monkeys during reversible lesions of medial cerebellar nuclei. J Neurophysiol 1981;46:828–38.ArticlePubMed
- 11. Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 1998;80:1911–31.ArticlePubMed
- 12. Ranalli PJ, Sharpe JA. Contrapulsion of saccades and ipsilateral ataxia: a unilateral disorder of the rostral cerebellum. Ann Neurol 1986;20:311–6.ArticlePubMed
- 13. Mossman S, Halmagyi GM. Partial ocular tilt reaction due to unilateral cerebellar lesion. Neurology 1997;49:491–3.ArticlePubMed
- 14. Baier B, Bense S, Dieterich M. Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain 2008;131:1445–54.ArticlePubMed
- 15. Brandt TH, Dieterich M. Different types of skew deviation. J Neurol Neurosurg Psychiatry 1991;54:549–50.ArticlePubMedPMC
Citations
Citations to this article as recorded by

- Free water imaging unravels unique patterns of longitudinal structural brain changes in Parkinson’s disease subtypes
Abigail E. Bower, Sophia J. Crisomia, Jae Woo Chung, Justin P. Martello, Roxana G. Burciu
Frontiers in Neurology.2023;[Epub] CrossRef





 KBS
KBS
 PubReader
PubReader ePub Link
ePub Link Cite
Cite