Abstract
- Case history of a 67-year-old man diagnosed with posterior benign paroxysmal positional vertigo (BPPV) with extremely long latencies after holding the Dix-Hallpike position for five minutes. Additional vestibular assessment indicated partial unilateral hypofunction. The patient had a history compatible with classic BPPV. This patient, however, did not have any positional nystagmus after doing standard positional testing. With extremely prolonged Dix-Hallpike testing (five minutes), the patient experienced nausea and vertigo. Concomitantly classic peripheral nystagmus was observed. After a total of seventeen treatments in a reposition chair a total relief of symptoms was obtained. The extremely long latencies observed in this patient were ascribed to otoconial adherence and/or otoconial size. This type of BPPV has not previously been described.
-
Keywords: Vestibular diseases; Benign paroxysmal positional vertigo; Vestibular function tests; Semicircular canals; Nystagmus, Pathological
-
중심단어: 양성돌발두위현훈, 전정기능검사, 반고리관
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is known as the most common cause of vertigo [1]. Under normal conditions, angular acceleration of the head initiates movement of endolymph in the semicircular canals (SCC), thereby causing deflection of hair cells embedded in the cupula [1]. In BPPV, otoconia in the utricle become detached and enter the SCC. This makes the SCC gravity-dependent and a change in head position therefore elicits rotational vertigo [1].
Otoconia displacement is most commonly seen in the posterior SCC (88.4%) [2]. Posterior SCC BPPV is diagnosed with the Dix-Hallpike test. BPPV is classified as either canalolithiasis or cupulolithiasis. Posterior canalolithiasis is common and causes upbeat and rotational nystagmus. The nystagmus typically lasts up to one minute and latency ranges from a few seconds up to 30 seconds [1,3]. Posterior SSC BPPV is successfully treated with the Epley maneuver. Over 90% resolves with one or two treatment sessions [4]. Canalolithiasis is thought to be easier to treat [1].
A mathematical model incorporating fluid dynamics indicates that latency is a result of several factors. Nystagmus occurs when the otoconia reaches the narrow point in the SCC, as otoconia in the ampulla has no effect on fluid pressure. The time it takes for the otoconia to reach this point depends on both its size and starting position in the ampulla. Extremely long latencies may be explained by otoconia adhering to the wall caused by viscous interactions between them. Based on these factors, the aforementioned model finds it prudent to hold each position for 30 seconds when performing canalith repositioning procedures (CRP) [5].
Clinical findings differing from the above might be due to more complicated pathologies such as multiple canal impairment, bilateral BPPV, subjective BPPV or even central pathology. Some cases of posterior BPPV are refractory to treatment [1,6,7]. In all cases, further investigation is required.
CASE REPORT
A 67-year-old male was referred to the Department of Otolaryngology, Head and Neck Surgery, Aalborg University Hospital, Denmark because of resilient BPPV. At the time of referral, the patient had been symptomatic for 21 months. Prior to referral, the patient had been diagnosed with BPPV involving different SCCs bilaterally and standard reposition maneuvers had been unsuccessful.
At the first visit, the patient was diagnosed with left posterior BPPV. At the second visit, the patient was diagnosed with left lateral BPPV. Both cases were treated successfully with the Thomas Richard-Vitton (TRV) reposition chair. On the third visit, the patient was still symptomatic but without any positional nystagmus. However, a fourth visit was scheduled, because the patient still experienced positional vertigo. At the fourth visit, the patient still complained of rotational vertigo when lying on his right side. He consistently experienced vertigo approximately five minutes after lying down on his right side. Therefore, an extended Dix-Hallpike test was performed. From the clinician’s viewpoint upbeat and counterclockwise rotational nystagmus was detected after holding the Dix-Hallpike position on the patient’s right side for five minutes. The nystagmus lasted approximately 30 seconds and a classic crescendo-decrescendo nystagmus was observed. The observed nystagmus was documented using videonystagmography (VNG) with Frenzel goggles. Based on the characteristic nystagmus elicited by the Dix-Hallpike test, the patient fulfills the recent diagnostic criteria of posterior BPPV canalolithiasis with the exemption of the extremely long latency observed [8].
The patient was diagnosed and treated in the TRV repositional chair. All treatments involved the Epley maneuver. A total of 16 treatments were performed. 12 of these treatments consisted of an extended Epley maneuver were kinetic forces were applied. When holding the first position, upbeat rotational nystagmus was detected with a latency of three to five minutes during each treatment. Usually nystagmus was elicited in the next position also, where the patient was rotated 45 degrees towards the opposite side. When rotating the patient to positions three to five, nystagmus was inconsistently present. See Fig. 1 for pictures showing each position.
During the period of treatment the patient became significantly less symptomatic. At the seventeenth visit the patient no longer had any complaints of vertigo and an extended right Dix-Hallpike test, where the position was held for ten minutes, did not elicit any nystagmus nor subjective nausea or vertigo.
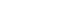
Brain MRI, otoneurological examination, and VNG testing with spontaneous nystagmus, gaze, smooth pursuit and saccades were all normal. Furthermore temporal bone MRI revealed no structural abnormalities of the inner ear. Also, neither cervical vestibular evoked myogenic potentials (cVEMP) nor ocular vestibular evoked myogenic potentials (oVEMP) were abnormal. Audiological testing revealed a symmetric bilateral noise induced hearing loss at 4 kHz but otherwise normal hearing. Video Head Impulse Test (v-HIT) indicated an impairment of the right posterior SCC with reduced VOR gain (0.57) and overt saccades (Fig. 2A). No impairment of the remaining five SCCs was found. Bithermal caloric testing was performed (Fig. 3). Nystagmus was induced bilaterally with the expected fast phase directions. However, nystagmus on the right side showed markedly less intensity with both warm and cold air compared to the left side (unilateral weakness 37%).
DISCUSSION
Initially the patient was treated for BPPV involving different SCCs. It is very likely that the patient initially had multiple-canal BPPV [9] affecting both the left and the right posterior SCCs. However, due to the extremely long latency of BPPV affecting the right posterior SCC, initially only BPPV of the left posterior SCC was diagnosed. This was treated successfully. During this treatment otoconia was probably moved from the left posterior SCC to the left lateral SCC [10]. Unpublished data from an ongoing study at the Department of Otolaryngology, Head & Neck Surgery, Aalborg University Hospital, Denmark shows that this is not uncommon when treating posterior BPPV with the TRV reposition chair. The BPPV involving the left lateral SCC was also treated successfully leaving only the atypical long latency BPPV of the right posterior SCC.
Vertigo is often due to inner ear disease, but central pathology is also a possibility. Some epidemiological studies show that 25% of patients complaining of vertigo is due to central pathology. Among the most common central pathologies causing vertigo are cerebrovascular disorders, migraine, multiple sclerosis and central positional vertigo/nystagmus (CPVN) [11]. CPVN was also considered but ruled out. Nystagmus seen with CPVN is typically purely vertical and without any latency [1,11]. However, this patient had classic peripheral nystagmus compatible with right-sided posterior canalolithiasis and had no prior history of inner ear disease. The extremely long latency observed was however very unusual and has not earlier been described. Nystagmus was only elicited by the Dix-Hallpike test and the Epley maneuver during which the patient consistently experienced vertigo and nausea without vomiting. Furthermore, a Brain MRI and several other tests and examinations ruled out CPVN and other central disorders [1,6,11].
Several factors affecting latency might explain the extremely long latencies observed. As mentioned in the introduction, latency is a result of otoconial size and starting position in the ampulla and also viscous interactions between the wall and the otoconia. Major structural abnormalities of the SCCs were ruled out after a normal temporal bone MRI-scan was done. Increased otoconial size increases viscous interactions between the wall and otoconia, thereby extending the time needed for otoconia to reach a point in the SCC where it exerts a fluid pressure sufficient enough to elicit nystagmus. It has been suggested, that BPPV refractory to treatment might be due to structural abnormalities in the SCC [7]. Larger otoconia fall more quickly and causes nystagmus with shorter latencies [5]. Knowing this, very small otoconia would theoretically be able to cause nystagmus with very long latencies. However, it seems unlikely that this mechanism is responsible for latencies lasting up to five minutes. Extremely long latencies caused by otoconial size might therefore be hypothesized to be due to very large particles or several small adhering particles resulting in partial canalith jamming. Incomplete blockage of the SCC might result in slow or no movement of the otoconia but still allow passage of endolymph. Very large particles or several small adhering particles could theoretically result in mechanical obstruction or increased viscous interactions with the wall for which reason breakdown of otoconia would be necessary for movement to occur. This could also possibly explain why this patient had nystagmus in the right Dix-Hallpike position and only in the following two positions when doing the Epley maneuver reposition in 45 degree intervals. Further reposition did not elicit any nystagmus probably because of partial canalith jamming resulting in no movement of otoconia. This might also explain the severity of the patient’s symptoms during CRP, as nystagmus is known to scale with the number of otoconia [5].
In rare cases an association between BPPV and abnormal v-HIT has been described [12,13]. However, the v-HIT examination of the right posterior SCC might not be truly pathological. Several of the head impulses have a gain value close to one, the overt saccades present quite late (more than 100 ms after the head turn has ended), and only four out of ten impulses contain overt saccades. To our knowledge no direct association between BPPV and caloric testing with significant unilateral weakness has been reported. The patient in this case had a distinct history of positional vertigo and nystagmus only elicited by the Dix-Hallpike test and the Epley maneuver. Therefore no obvious association between these findings can be made. To rule out imprecise measurements complete v-HIT and caloric testing was repeated. V-HIT showed normal gain values and no pathological saccades regarding all six SCCs (Fig. 2B). Repeated caloric testing remained pathological with right-sided hypofunction. Because different frequencies are tested with v-HIT and caloric testing, it is possible to get conflicting results even though both tests evaluate the function of the lateral SCCs. Right-sided hypofunction cannot theoretically influence the diagnostics of posterior BPPV, as different SCCs with separate innervation are affected.
Based on this discussion we speculate that the patient’s vertigo was caused by BPPV and that the extremely long latency observed was a result of otoconial size and/or otoconia adhering to the inner SCC wall. In patients with a history highly suspective of BPPV, but with vertigo presenting minutes after a positional change, one should bear in mind, that an extended Dix-Hallpike test might reveal atypical BPPV occurring after extremely long latencies.
ARTICLE INFORMATION
-
No potential conflict of interest relevant to this article was reported.
Fig. 1.Illustrations of Epley maneuver treatment with the Thomas Richard-Vitton reposition chair reposition chair. Numbers one through six indicate initial right Dix-Hallpike position and subsequent repositions with 45 degree turns away from the affected side.

Fig. 2.Video head impulse test (v-HIT) of the right posterior semicircular canal (SCC) and the left anterior SCC (left anterior right posterior, LARP). (A) Initial v-HIT, right posterior SCC with gain 0.57 and overt saccades. (B) Repeated v-HIT, right posterior SCC with gain 0.97 and no pathological saccades.
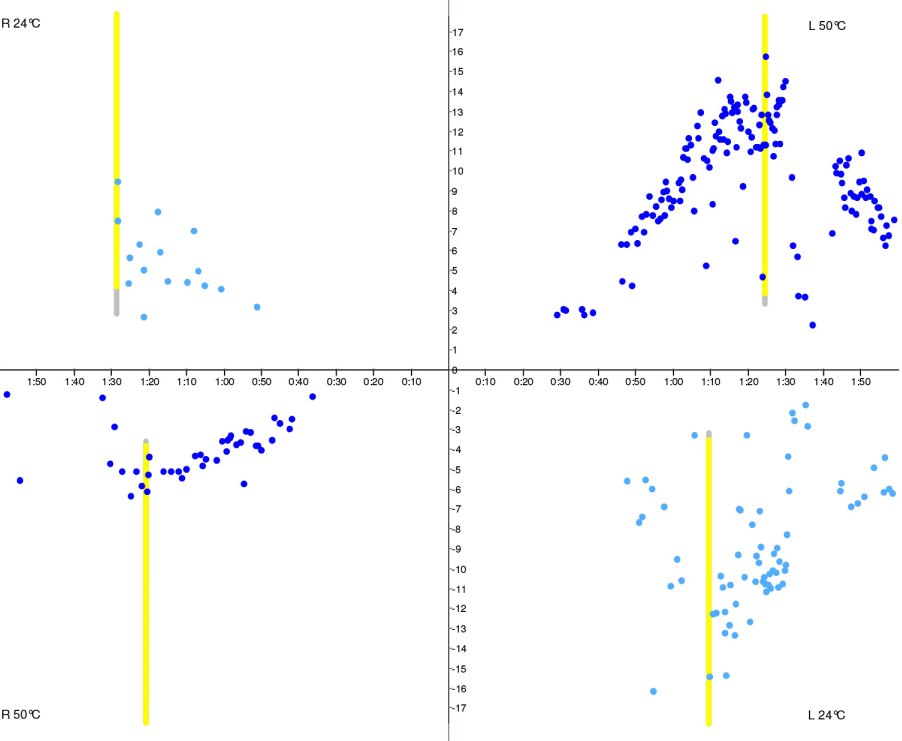
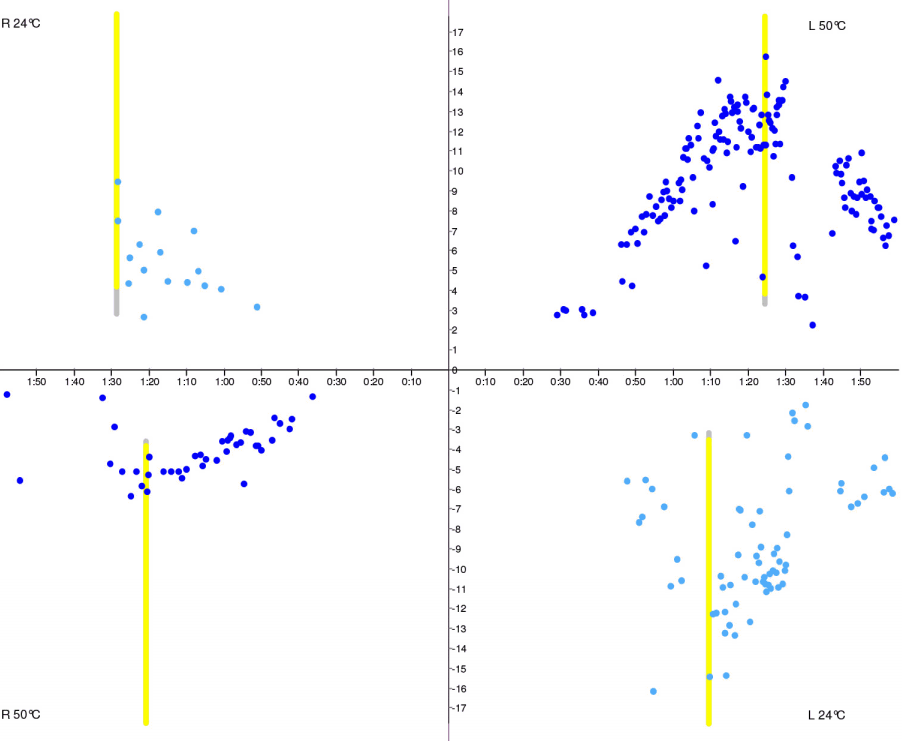
Fig. 3.Caloric test butterfly graph. Right-sided hypofunction. Vertical axis, slow phase velocity (deg/sec); horizontal axis, time (minutes).
REFERENCES
- 1. Buki B, Tarnutzer AA. Vertigo and dizziness. Oxford: Oxford University Press; 2014.
- 2. Soto-Varela A, Santos-Perez S, Rossi-Izquierdo M, Sanchez-Sellero I. Are the three canals equally susceptible to benign paroxysmal positional vertigo? Audiol Neurootol 2013;18:327–34.ArticlePubMed
- 3. Brandt T, Steddin S. Current view of the mechanism of benign paroxysmal positioning vertigo: cupulolithiasis or canalolithiasis? J Vestib Res 1993;3:373–82.PubMed
- 4. Sealy A. Vestibular assessment: a practical approach. Occup Med (Lond) 2014;64:78–86.ArticlePubMed
- 5. Hain TC, Squires TM, Stone HA. Clinical implications of a mathematical model of benign paroxysmal positional vertigo. Ann N Y Acad Sci 2005;1039:384–94.ArticlePubMed
- 6. Bhattacharyya N, Baugh RF, Orvidas L, Barrs D, Bronston LJ, Cass S, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 2008;139:S47–81.ArticlePubMed
- 7. Kaski D, Bronstein AM. Epley and beyond: an update on treating positional vertigo. Pract Neurol 2014;14:210–21.ArticlePubMed
- 8. von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, et al. Benign paroxysmal positional vertigo: Diagnostic criteria. J Vestib Res 2015;25:105–17.ArticlePubMed
- 9. Tomaz A, Gananca MM, Gananca CF, Gananca FF, Caovilla HH, Harker L. Benign paroxysmal positional vertigo: concomitant involvement of different semicircular canals. Ann Otol Rhinol Laryngol 2009;118:113–7.ArticlePubMed
- 10. Foster CA, Zaccaro K, Strong D. Canal conversion and reentry: a risk of Dix-Hallpike during canalith repositioning procedures. Otol Neurotol 2012;33:199–203.ArticlePubMed
- 11. Karatas M. Central vertigo and dizziness: epidemiology, differential diagnosis, and common causes. Neurologist 2008;14:355–64.ArticlePubMed
- 12. Heidenreich KD, Beaudoin K, White JA. Can active lateral canal benign paroxysmal positional vertigo mimic a false-positive head thrust test? Am J Otolaryngol 2009;30:353–5.ArticlePubMed
- 13. Mangabeira Albernaz PL, Zuma EMFC. The video head impulse test. Acta Otolaryngol 2014;134:1245–50.ArticlePubMed
Citations
Citations to this article as recorded by

- Upbeat and Direction-Changing Torsional Nystagmus While Straight Head Hanging: A New Sign of Benign Paroxysmal Positional Vertigo Involving Bilateral Posterior Semicircular Canals
Hyun-Jae Kim, Sang Jin Park, Ji-Soo Kim
Journal of Clinical Neurology.2024; 20(1): 100. CrossRef









 KBS
KBS
 PubReader
PubReader ePub Link
ePub Link Cite
Cite