Acute Bilateral Vestibulopathy with Concomitant Progressive Deterioration of Binaural Hearing
Article information
Abstract
Abstracts
Bilateral vestibulopathy is a condition with vestibular hypofunction of both inner ears. Patients with this diagnosis will often complain of dizziness and/or imbalance in darkness and when walking in uneven terrain and will often also experience oscillopsia. Predominant etiology is idiopathic. A 73-year old man with complaints of dizziness for 2 days. Objective findings included spontaneous nystagmus, a positive Romberg test with eyes closed, and a pathological video head impulse test. Initial audiometry only revealed bilateral presbycusis. Following gradual non-complete remission of vertiginous symptoms, the patient was discharged and scheduled for follow-up. The patient was later readmitted due to gradual progressive bilateral hearing deterioration alongside persisting vertiginous symptoms. Various additional tests all came out negative, and the condition was classified as idiopathic acute bilateral vestibulopathy with concomitant progressive deterioration of binaural hearing. The patient was later referred to bilateral cochlear implantation. Acute monosymptomatic bilateral vestibulopathy is difficult to diagnose, as it requires very specific tests that are not routinely done by neurologists. Acute bilateral vestibulopathy with concomitant progressive deterioration of binaural hearing leading to bilateral anacusis is indeed so rare that it has not been possible to find any literature describing a similar case.
INTRODUCTION
Vestibulopathy is a condition with complete loss of or compromised function of one or more structures within the vestibulum (the sensory organ within the inner ear that detects linear and angular accelerations of the body and thereby is one of the major contributors to maintaining balance). The paired vestibular end organs each consist of five separate organs; three semicircular canals (SCCs) and two otolith organs (saccule and utricle). Patients with the compromised vestibular function will often complain of dizziness and/or imbalance. However, these symptoms are non-localizing because disease/hypofunction of other sensory systems may also cause similar symptomatology, as the balance is maintained by an interaction between sensory signals from both the vestibulum, the eyes as well as pro-prioceptive input which is then integrated and processed in the brainstem, the cerebellum, and the cerebrum. Traditionally, examinations and accurate diagnostics within this group of patients have been extremely difficult. However, within the last decade, both diagnostics and treatments for this group of patients have improved significantly.
Bilateral vestibulopathy refers to a condition where the vestibular function, often acute, is compromised bilaterally. A condition with hypofunctioning vestibular organs is very disabling and causes pronounced problems with maintaining balance— especially in the dark and when walking in uneven terrain. Oscillopsia, which is a visual disturbance in which objects in the visual field appear to oscillate, often accompanies this condition. It is caused by a hypofunctioning or absent vestibulocular reflex (VOR) bilaterally [1].
Bilateral vestibulopathy is a heterogeneous condition with a wide range of etiologies, e.g. congenital, toxic, infectious, autoimmune, neurodegenerative, genetic, vascular, neoplastic, or traumatic. However, idiopathic is by far the most predominant cause and accounts for up to 50% of the total prevalence [1,2]. There may be concomitant audiological symptoms [1,2], although this is very rare within the idiopathic group of patients [1].
CASE REPORT
A 73-year-old male was referred following an acute onset of vertigo with a 2-day history of progressive, constant, rotational dizziness. Accompanying symptoms included nausea, vomiting, headache, and a 5-day history of right-sided hearing loss without tinnitus. Objective findings included spontaneous, horizontal, and right-beating nystagmus that complied with Alexander's Law (with gaze toward the side of the fast phase, nystagmus amplitude and frequency increased, and both decreased with gaze toward the slow phase side).
Due to the severity of the patient's vertigo, the information gained from the initial otoneurologic examination was very limited. The following day, a more extensive and partly successful examination was performed now showing a fast phase shift of the patient's horizontal nystagmus that did still comply with Alexander's Law. The patient's blood samples showed an increase in C-reactive protein and leukocytes, and labyrinthitis was considered. However, labyrinthitis and also central nervous system infection were later ruled out.
Four days after admission, a thorough examination was performed with the following major findings; Romberg test on foam was positive with eyes closed only. A complete video head impulse test (vHIT) showed hypofunction of all six SCCs. Ocular vestibular evoked myogenic potentials (oVEMP) and cervical vestibular evoked myogenic potentials (cVEMP) testing were abnormal with no myogenic potentials bilaterally. Subsequent conclusion was therefore bilateral dysfunction of the otolithic organs as well as all SCCs (Fig. 1). Cerebral magnetic resonance imaging (MRI) was normal.
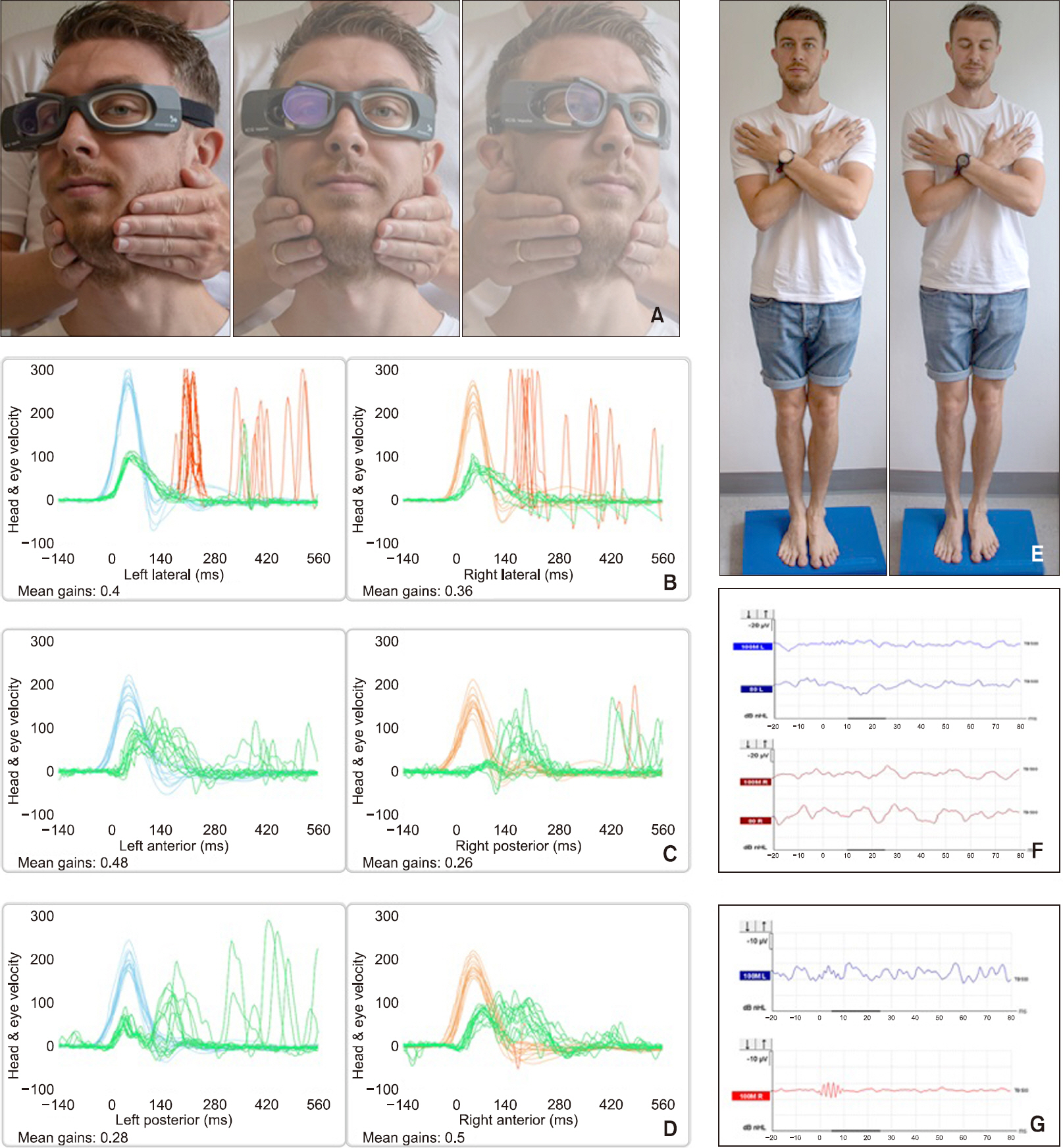
Clinical examinations. (A) Video head impulse testing of the lateral semicircular canals. (B) Test results following lateral semicircular canal (SCC) testing with gain values of 0.4 and 0.36 on the left and right side respectively with accompanying pathological overt saccades bilaterally. (C) Test results following left anterior/right posterior (LARP) SCC testing with gain values of 0.48 and 0.26 on the left and right side respectively. LA testing with accompanying pathological covert saccades (not recognized by the software) and RP testing with accompanying pathological overt saccades. (D) Test results following right anterior/left posterior (RALP) SCC testing with gain values of 0.28 and 0.5 on the left and right side respectively with accompanying pathological saccades not recognized by the software bilaterally (LP, overt saccades; RA, mixed saccades). X-axis, time in milliseconds; Y-axis, mirrored view of head and eye velocities in degrees per second; light blue line, head velocity on the left side; orange line, head velocity on the right side; red line, pathological saccades recognized by the accompanying software; green line, eye velocities on both the left and right side (B-D).(E) Romberg test on foam with eyes open and closed. (F) Cervical vestibular evoked myogenic potentials with no P13/P23 potentials bilaterally. (G) Ocular vestibular evoked myogenic potentials with no P10/P15 potentials bilaterally.
After 1 week of hospitalization with progressive subjective improvement of vertiginous symptoms, the patient was discharged and scheduled for an outpatient follow-up.
One and a half months following primary hospitalization, the patient was readmitted due to progressive bilateral hearing deterioration (bilateral anacusis developed) alongside persistent hypofunctioning vestibular organs bilaterally.
Several consecutive audiometries were performed. Initially, these exclusively displayed bilateral symmetrical sensorineural hearing loss compatible with presbycusis. Subsequent audiometries showed progression of inner ear hearing loss on the right side only and later also on the left side (Fig. 2).

Consecutive audiometries. (A) Initial tone audiometry revealed symmetrical bilateral presbycusis, a small symmetrical conductive bilateral hearing loss, and a bilateral noise-induced hearing loss at 4 kHz. Speech discrimination scores were down approximately 50% bilaterally. (B) Bone conduction thresholds (inner ear involvement) went down bilaterally. (C) The hearing loss progressed and anacusis was seen on the right side and profound hearing loss on the left side. (D) Finally, anacusis was diagnosed bilaterally. There is 1 day between the audiograms displayed in Fig. 2A and 2B, one and a half months between the audiograms displayed in Fig. 2B and 2C, and 2 weeks between the audiograms displayed in Fig. 2C and 2D. The degree of subjective hearing loss was in accordance with the audiometric tests performed throughout the audiometric follow-up period of approximately 2 months.
The vestibular function tests in terms of complete vHIT testing remained unchanged throughout the follow-up period with pathological low gain values with concomitant pathological saccades for all six SCCs. The additional and more extensive vestibular tests (oVEMPS and cVEMPs) were not repeated during the follow-up period as vestibular symptoms did not change during the follow-up period.
Cerebral MRI, computed tomography (CT) scans of the thorax, abdomen, and pelvis, CT with angio sequences, chest X-ray, human immunodeficiency virus test, and syphilis test were performed. To exclude any underlying neurological, infectious, or rheumatic disorder, relevant blood tests and lumbar puncture were performed as well. All tests came out negative.
High-dose steroid therapy was subsequently given for 3 days because of suspected autoimmune disease (potentially autoimmune inner ear disease, AIED). This patient received 1-g methylprednisolone daily for 3 days.
After this treatment was completed, additional audiometry revealed unchanged bilateral anacusis, and the condition was classified as idiopathic acute bilateral vestibulopathy with concomitant progressive deterioration of binaural hearing. Due to a pronounced loss of communicative skills, the patient was found eligible for bilateral cochlear implantation just 2 months after the initial admission. The patient has now, sequentially, been implanted with cochlear implants (CI) bilaterally. The patient has experienced partial recovery of his vertigo due to central compensation by means of adaptation, substitution, and/or habituation and has been granted lifelong vestibular rehabilitation. The patient can go to work and unfold physically even though both his inner ears remain hypofunctioning. Written informed consent was obtained for the detailed data of this case report.
DISCUSSION
From a clinical perspective, this case report is very interesting because of complete bilateral inner ear involvement exclusively with the rare combination of initial acute bilateral vestibulopathy combined with gradual progressive bilateral hearing deterioration.
Today, many new examination modalities exist, each providing objective evaluation of the function of all parts of the inner ear individually. Despite these groundbreaking diagnostic improvements, a large proportion of the diseases which affect the function of the inner ear are still to be classified as idiopathic. Thus, it is not unlikely that this disease has an unknown etiology, e.g. AIED. Recommended AIED treatment is prednisone of 1 mg/kg/day for up to 4 weeks [3,4]. This patient, however, received 1-g methylprednisolone daily for only 3 days. If more prolonged steroid treatment had been performed, theoretically, it might have induced short-term improvement of cochlear function. However, it should be stressed that the symptomatology in this case report does not fit classic AIED with selective cochlear involvement only.
In our experience, patients with bilateral vestibular function require lifelong vestibular rehabilitation in order to maintain optimal central compensation by means of adaptation, sub-stitution, and/or habituation.
The patient received the best possible contemporary treatment available in Denmark. However, despite a good prognosis in terms of future communication skills following bilateral CI surgery, the patient will depend on lifelong vestibular rehabilitation in order to maintain reasonable and optimal balance skills. Future patients may face a better prognosis as treatment of bilateral vestibulopathy is also improving immensely. Experimental implantation of vestibular implants and combined cochlear and vestibular implants have been done. Results have been promising and this treatment modality might be offered to patients with a similar case history in the near future [5].
Acute monosymptomatic bilateral vestibulopathy is difficult to diagnose. Neurological examination will most likely be described as being normal, because this diagnosis requires specific examinations that are not routinely done by neurologists. A diagnosis of bilateral vestibulopathy requires an absent or significantly impaired VOR. Diagnostic criteria include (1) bilateral vHIT with a horizontal angular VOR gain on both sides below 0.6; and/or (2) caloric testing where the sum of the maximal peak velocities of the slow phase caloricinduced nystagmus for stimulation with warm and cold water on each side is less than 6°/sec; and/or (3) rotational chair testing where the horizontal angular VOR gain is below 0.1 upon sinusoidal stimulation (0.1 Hz, Vmax=50°/sec) and/or a phase lead of >68° (time constant of <5 seconds). Complementary tests may also include dynamic visual acuity, Romberg test and cVEMP and oVEMP testing [6]. The majority of the patients will also complain of visual disturbances caused by an accompanying oscillopsia. This case report was chosen for the abovementioned reasons and the condition is indeed so rare that it has not been possible to find any literature describing a similar case.
Notes
No potential conflict of interest relevant to this article was reported.
