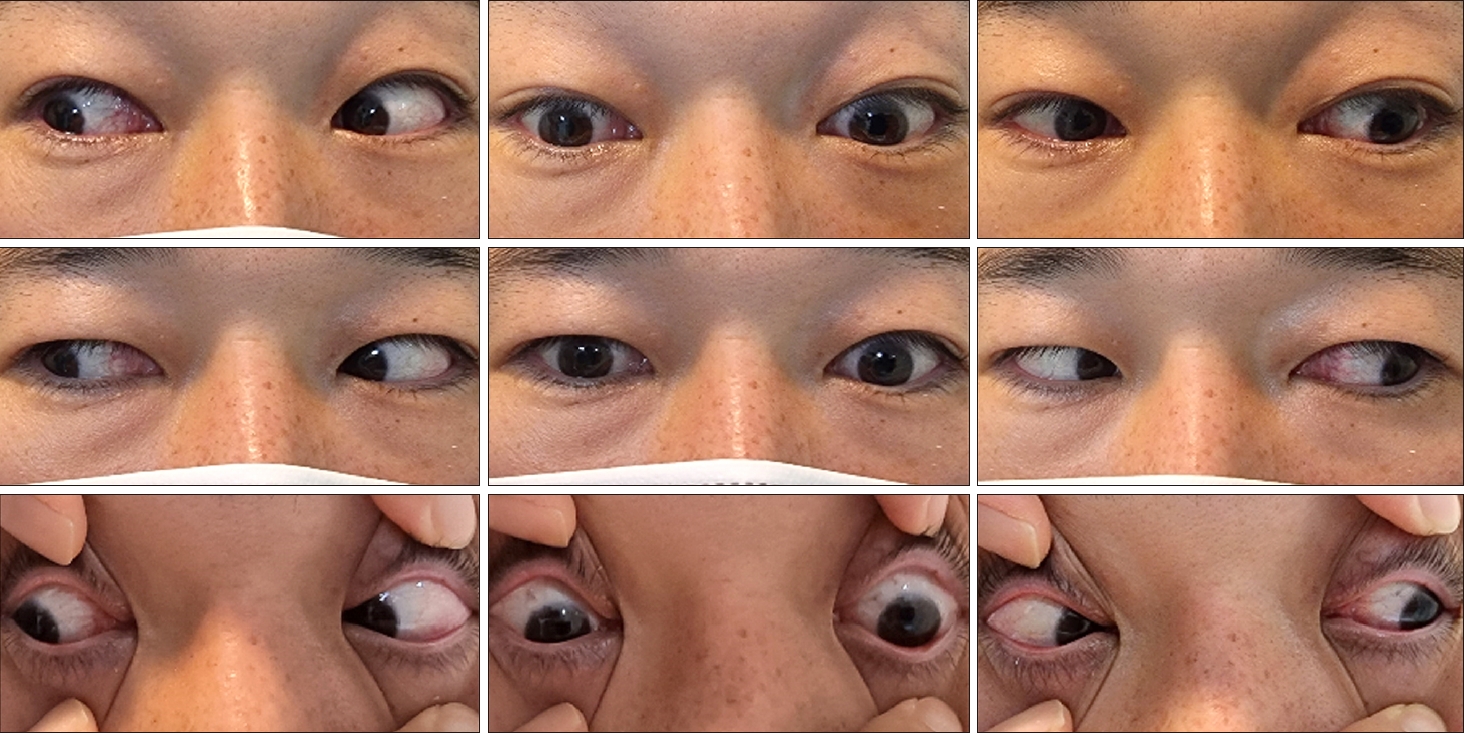
시고정 유발 편측시소안진
Visual Fixation-Induced Hemi-Seesaw Nystagmus
Article information
Trans Abstract
Seesaw nystagmus (SSN) is characterized by conjugate torsional nystagmus with opposite vertical components in the two eyes. The waveform may be pendular or jerk (hemi-seesaw nystagmus, HSSN), in which the slow phase corresponds to one half-cycle and the quick phase to the other. Pendular SSN and HSSN have distinct clinical presentations and underlying causes. The pathophysiology of pendular SSN may be instability of visuovestibular interactions, while the underlying mechanism for HSSN may be related to the ocular tilt reaction or an imbalance in vestibular pathways. We report a patient with HSSN due to unilateral mesodiencephalic infarction that becomes apparent during visual fixation only.
INTRODUCTION
Hemi-seesaw nystagmus (HSSN) is characterized by conjugate torsional jerky nystagmus with opposite vertical components in the two eyes [1]. HSSN has been reported in unilateral brainstem lesions involving the interstitial nucleus of Cajal (INC) or medial longitudinal fasciculus (MLF) [1-3]. We report a patient with a unilateral INC lesion who showed HSSN only during visual fixation.
CASE REPORT
A 36-year-old male presented with sudden-onset vertical diplopia, dizziness, and dysarthria. He had a history of arrhythmogenic right ventricular dysplasia. An ocular motor examination revealed bilateral upward gaze palsy with partial impairment of downward gaze, which was not resolved by the doll’s head maneuver (Fig. 1). Horizontal gaze was intact and the pupils were equal and reactive to light. Eye-movement recording using three-dimensional video-oculography showed HSSN during visual fixation, consisting of intorsional upbeat nystagmus in the right eye that occurred synchronously with extorsional downbeat nystagmus in the left eye (counterclockwise from the patient’s perspective) (Fig. 2, Supplementary Video 1). The frequency of the nystagmus was consistent at 2 Hz. The torsional component was larger in the left eye than the right eye, while the vertical component was larger in the right eye. The slow phases of the nystagmus showed exponentially decreasing waveforms with the exception of the vertical component of the left eye showing linear waveforms. The vertical and torsional components decreased during leftward gaze and changed to upbeat and counterclockwise during rightward gaze. The nystagmus disappeared completely in darkness when visual fixation was removed (Fig. 2, Supplementary Video 1). Vertical saccades and smooth pursuit were impaired due to vertical gaze palsy. Testing the subjective visual vertical (SVV) showed no tilt during binocular viewing (2.2°; normal range, ‒3.0° to 3.0°; a negative value indicates a leftward tilt), and fundus photography did not reveal ocular torsion in either eye. The vestibulo-ocular reflex (VOR) gains for horizontal canals were normal in video head impulse tests, but vertical head impulses could not be performed due to vertical gaze palsy. Magnetic resonance imaging of the brain revealed acute infarction in the left medial thalamus and upper midbrain (Fig. 3). Due to the presence of atrial fibrillation on an electrocardiogram, the patient was treated with an oral anticoagulant. The HSSN resolved a day later, but convergence-retraction nystagmus appeared. A 2-month follow-up examination showed resolution of the vertical gaze palsy and convergence-retraction nystagmus.
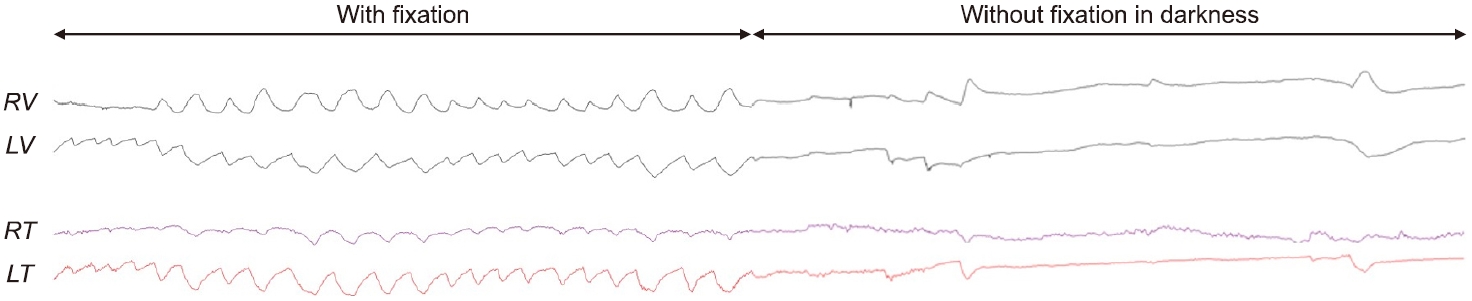
Eye-movement recordings. Three-dimensional video-oculography shows hemi-seesaw nystagmus (HSSN) during visual fixation, consisting of counterclockwise torsional nystagmus (from the patient’s perspective) with upbeat nystagmus in the right eye and downbeat nystagmus in the left eye. The HSSN disappears completely in darkness. RV, vertical position of the right eye; LV, vertical position of the left eye; RT, torsional position of the right eye; LT, torsional position of the left eye.
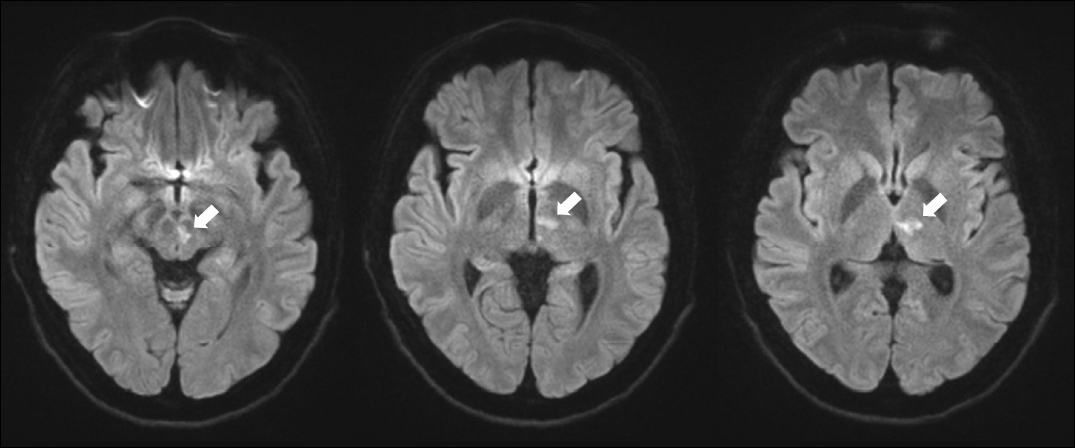
Magnetic resonance imaging scans of the brain revel an acute infarction (arrows) at the left medial thalamus and upper midbrain.
This study followed the tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (No. 05-2023-002). Written informed consent was obtained for publication of this case report and accompanying images.
DISCUSSION
The INC, located at the dorsomedial midbrain tegmentum is known to be responsible for generating vertical and torsional eye-position signals [1]. It has extensive afferent and efferent pathways to the vestibular nuclei that are conveyed via the MLF. Therefore, unilateral brainstem lesions involving the INC or along its afferent/efferent pathways in the MLF induce an imbalance of vestibular signals such as the ocular tilt reaction (OTR), which may lead to HSSN [1-3]. This was supported by an experimental finding that muscimol inactivation caudal to the INC induced HSSN [4]. Thus, the pathophysiology of HSSN may be associated with the OTR due to unilateral interruption of otolith graviceptive pathways from the labyrinth to the INC [1]. Because the torsional-vertical components of HSSN were in the direction opposite to the OTR, it was hypothesized that HSSN is a compensatory phenomenon for the OTR [1,2]. However, our patient did not show abnormal SVV tilt or ocular torsion despite the presence of a unilateral INC lesion. Normal static and dynamic otolith functions have also been reported in Joubert syndrome with HSSN [5]. Furthermore, not all patients with OTR have HSSN. These findings suggest that other mechanisms such as role of the vertical-torsional neural integrator are crucial for generating HSSN in a unilateral INC lesion [1,5].
It was particularly interesting that the HSSN in our patient disappeared completely in darkness when visual fixation was removed, suggesting that visual input is involved in the generation of this type of nystagmus. In fact, pendular seesaw nystagmus (SSN) has been reported in association with visual impairments such as bitemporal hemianopsia [6,7]. This may result from the miscalibration of retinal error signals and compromise of visuovestibular adaptive mechanisms via the accessory optic system (AOS) [8]. During head movements, retinal error signals are conveyed via the AOS to the inferior olivary nucleus, which in turn projects climbing fibers to the floccular Purkinje cells of the cerebellum, and then cerebellar Purkinje cells mediate adaptation of the VOR to minimize retinal error signals [8]. Thus, disruption of visual inputs and the resultant instability of visuovestibular interactions may be the underlying mechanism for pendular SSN [6,7]. Although pendular SSN and HSSN have distinct clinical presentations and underlying causes, the visual modulation of HSSN in our patient may be explained by the same mechanism. The AOS comprises three paired terminal nuclei at the mesodiencephalic border, with each pair processing visual information for one of three cardinal rotational axes [8]. Among them, the dorsoterminal nucleus (DTN) is located around the INC along with the nucleus of the optic tract. In our patient, simultaneous damage of the DTN could have blocked the transmission of retinal error signals caused by binocular vertical misalignment, leading to the visual modulation of HSSN. A rapid resolution of HSSN within a day may reflect a rapid recovery of visuovestibular adaptive mechanisms from transient ischemia of the DTN.
Supplementary materials
Supplementary Video 1 can be found via https://doi.org/10.21790/rvs.2023.22.1.19.
Eye-movement recording using three-dimensional video-oculography shows hemi-seesaw nystagmus (HSSN) during visual fixation, consisting of counterclockwise torsional nystagmus (from the patient’s perspective) with upbeat nystagmus in the right eye and downbeat nystagmus in the left eye. The HSSN disappears completely in darkness. RV, vertical position of the right eye; LV, vertical position of the left eye; RT, torsional position of the right eye; LT, torsional position of the left eye.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING/SUPPORT
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
AUTHOR CONTRIBUTIONS
Conceptualization: All authors; Data curation: HSK; Investigation: EHO; Funding acquisition: JHC; Supervision: JHC; Writing-Original Draft: HSK; Writing-Review & Editing: All authors. All authors read and approved the final manuscript.