Abstract
- Because numerous important nuclei and white matter tracts are concentrated in the narrow midbrain, the tiny lesion can result in various symptoms. The anatomy of the neural network and related structures in the midbrain is complex. The most frequent clinical manifestation of lesions involving the midbrain is an eye movement disorder associated with a nuclear or fascicular origin. We have described patients with acute midbrain stroke, characterized by rare neuro-ophthalmologic manifestations, which neurologists should consider during diagnostic investigations. Case 1 showed internuclear ophthalmoplegia with Horner syndrome. In case 2 showed isolated oculomotor palsy. The third patient presented Parinaud syndrome with an unusual lesion location. Notably, patients with midbrain infarction may present with specific signs and symptoms that are compatible with mesencephalic localization. The isolated or combined neuro-ophthalmologic signs and symptoms should be interpreted in the complex anatomical context described here and investigated by immediate brainstem neuroimaging analyses and careful neurologic examinations.
-
Keywords: Brain stem infarctions; Cerebral infarction; Magnetic resonance imaging; Mesencephalon; Ocular motility disorder
-
중심단어: 뇌간 뇌졸중, 뇌경색증, 자기공명영상, 중간뇌, 안구운동장애
INTRODUCTION
The mesencephalon is the upper brainstem bordered rostrally by the diencephalon and situated caudally adjacent to the pons [1]. Because of its critical location relative to the cerebrum, cerebellum, and lower brainstem, the mesencephalon contains multiple ascending/descending tracts that relay the control of body function among these structures [2]. It is important in the processing of mental status and motor-sensory interactions, as well as in ocular motility control [3]. However, most of the corresponding tracts and nuclei are not well discriminated by current neuroimaging modalities. Nevertheless, a detailed understanding of the relevant anatomy is necessary for better com-prehension of the precise locations of these crucial midbrain structures and their functions [2]. Acute ischemic strokes involving the midbrain may result in a distinct set of symptoms, depending on the region affected by the lesion. Some lesions can result in subsequent neuro-ophthalmologic manifestations. Importantly, the close proximity caused by the narrow space between these midbrain structures can lead to overlapping clinical symptoms that vary among involved regions [4].
A review of midbrain anatomy illustrated by uncommon midbrain syndromes may be useful for neurologists who seek to connect neuroimaging findings with clinical manifestations. Here, we describe three patients with rare midbrain infarctions that manifested as internuclear ophthalmoplegia (INO), isolated oculomotor nerve palsy, and Parinaud syndrome.
CASE REPORT
Ethical approval for this report was granted in accordance with national requirements, and the need for written informed consent was waived by the Institutional Review Board of Jeju National University Hospital (No. 2019-03-014). The figures have been edited by authors with maximum dedication for the protection of patient's personal information, and the most prominent expression of ocular movement disorders.
1. Case 1
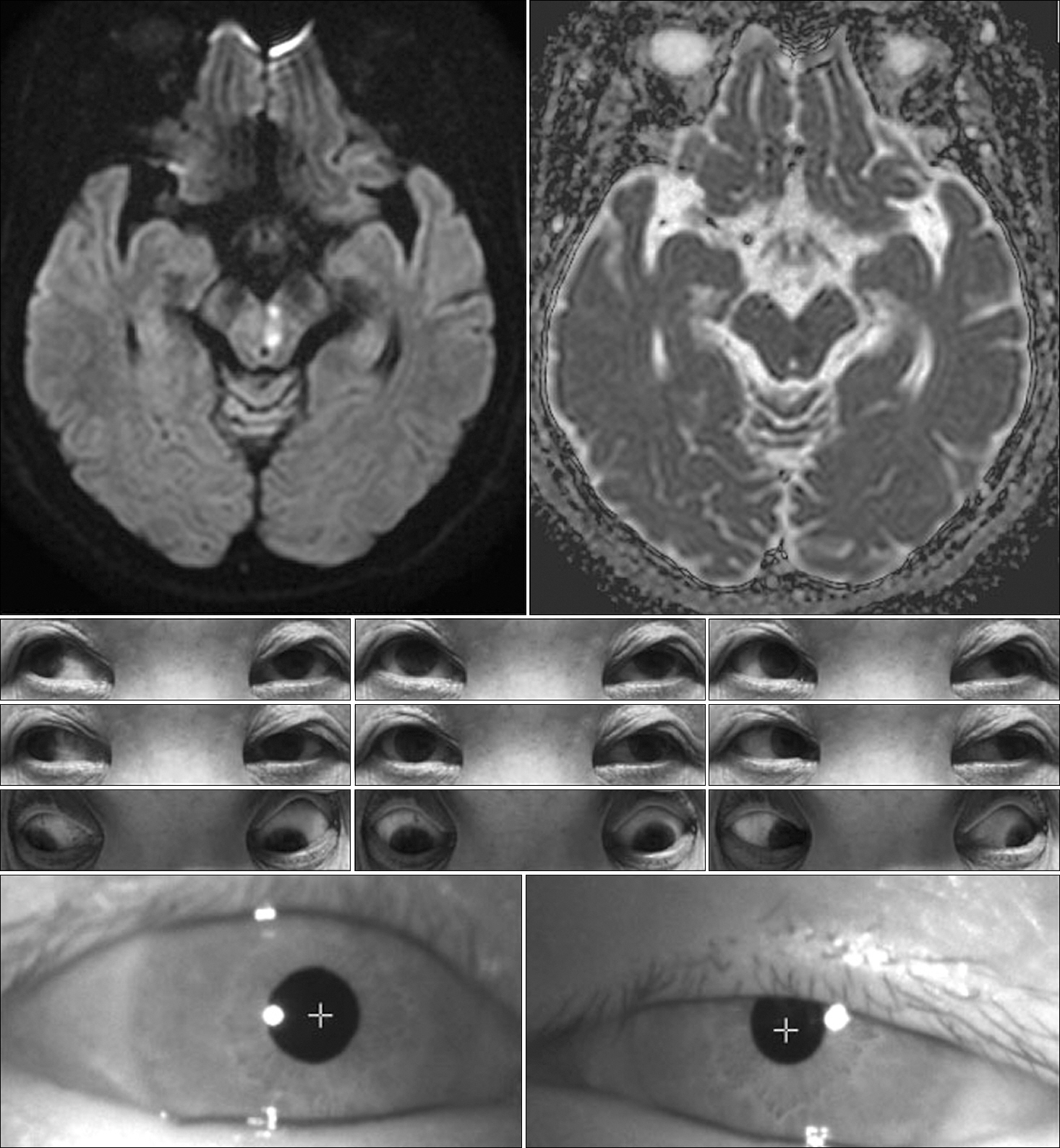
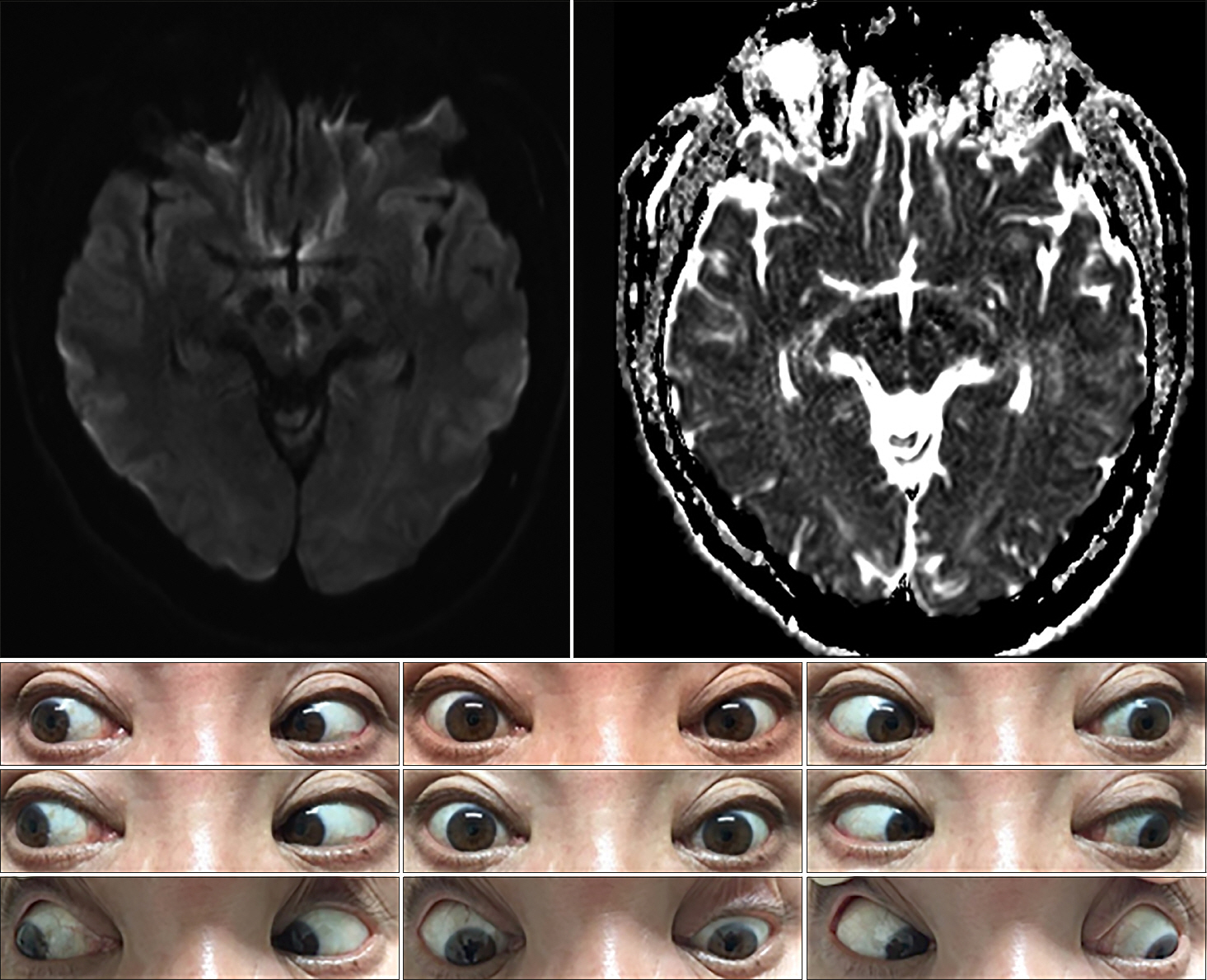
A 75-year-old woman was admitted to our hospital for sudden onset of binocular horizontal diplopia. Initial test of ocular motility demonstrated the horizontal diplopia to be subjectively aggravated during the right gaze. Neurologic examination revealed outward deviation of the left eye at the primary neutral position. Furthermore, subtle left ptosis was evident with miosis and limited adduction in the left eye. Initial test of ocular motility demonstrated the horizontal diplopia to be subjectively aggravated during the right gaze. Also, horizontal nystagmus of the right eye was markedly provoked during the right lateral gaze. The convergence was possible on the indication of a near target despite the limit of voluntary adduction in the left eye. Vertical eye movements were preserved and there was no clinical evidence of ocular tilt reaction (OTR). Diffusion-weighted images demonstrated a high-signal intensity lesion in the paramedian midbrain at the inferior colliculus level and ventrolateral to the cerebral aqueduct (Fig. 1). No associated vascular steno-occlusive lesion was identified. The Horner syndrome and diplopia had disappeared one month later, although a slight adduction delay persisted in the left eye.
Fig. 1.Brain magnetic resonance imaging shows restricted diffusion in the left inferior midbrain, immediately ventral to the cerebral aqueduct with hypointensity on apparent diffusion coefficient images. The nine-gaze photograph shows limited adduction of the left eye, unilateral partial ptosis, and miosis.

2. Case 2
A 67-year-old man developed sudden dizziness and diplopia. He had a history of hypertension and heavy smoking. Examination showed isolated complete left oculomotor nerve palsy with ptosis and mydriasis. In the primary neutral position, his left eye deviated outward and downward. The results of other routine neurologic examinations were normal. Brain magnetic resonance imaging (MRI) demonstrated a small infarct in the left upper midbrain (Fig. 2). The patient's oculomotor nerve palsy was more improved one month after the onset of the initial symptoms.
Fig. 2.Brain magnetic resonance imaging shows restricted diffusion in the ventromedial midbrain with hypointensity on apparent diffusion coefficient images. The nine-gaze photograph shows complete left oculomotor nerve palsy with ptosis and mydriasis. The left eye demonstrated limitations in adduction, elevation, and depression. (In the nine-gaze photo, the supraduction limitation of the left eye seems to be incomplete, but on the initial neurologic examination, a recognizable paralysis was confirmed.)
3. Case 3
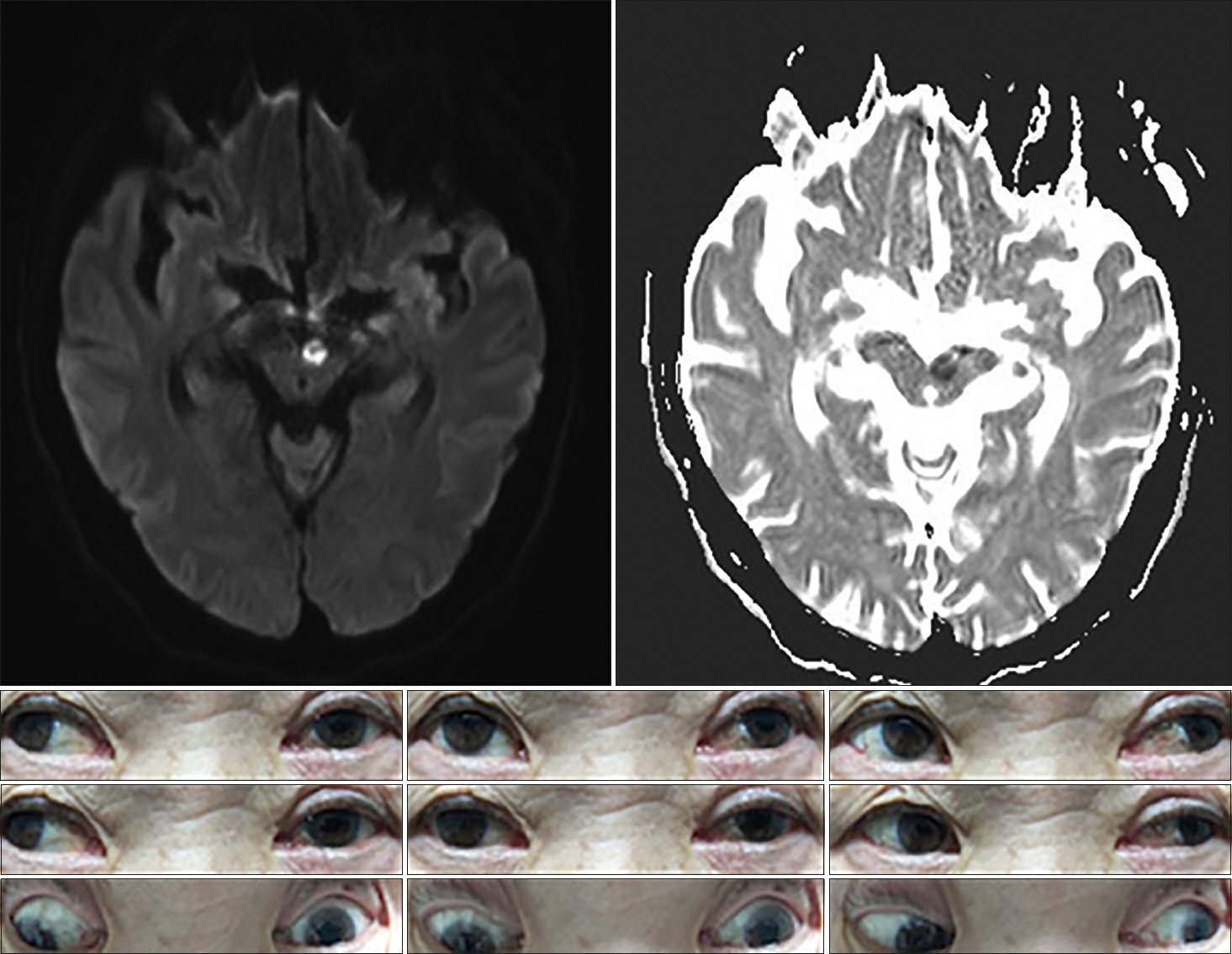
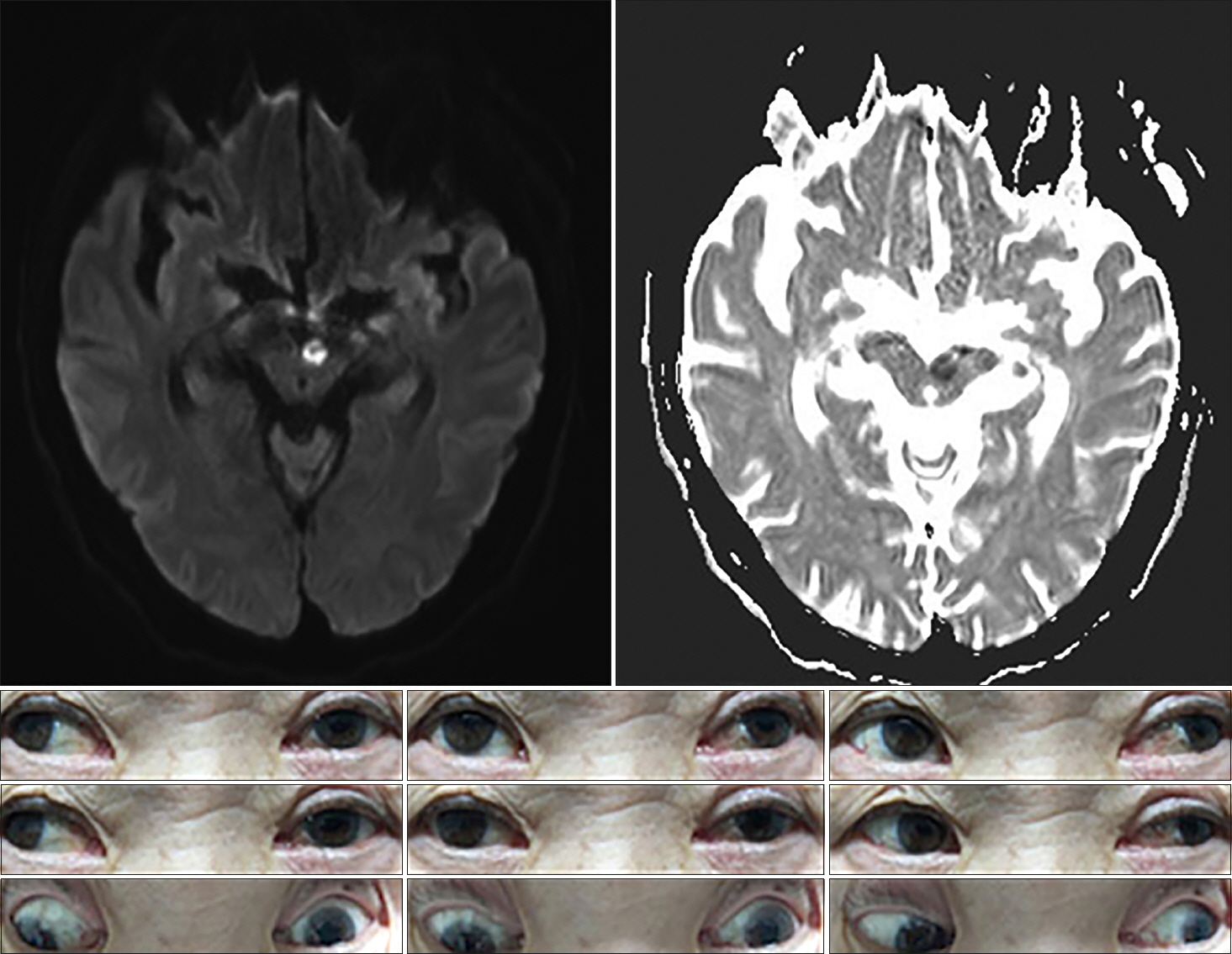
A 61-year-old man presented with diplopia and dizziness on awakening. He had no significant medical history (e.g., recent trauma, myasthenia gravis, or thyroid disease). On examination, he exhibited vertical gaze paralysis with torsional nystagmus (more robust in the left eye). He also exhibited classical convergence-retraction nystagmus during attempted upward gaze. Vertical gaze palsy could be overcome near the midline through the doll's eye maneuver, which suggestive of the spared vertical vestibuloocular reflex (VOR). Although there was a mild limit on downward saccades, downgaze was relatively preserved. OTR did not significantly reveal on initial examination. He showed mild light-near dissociation, whereby the pupils responded briskly with convergence but responded poorly to light. The remaining neurologic examination findings were normal, as were the cranial nerve examination findings. MRI revealed infarction in both paramedian dorsal midbrain (more prominent on the left side) on a diffusion-weighted image (Fig. 3).
Fig. 3.Brain magnetic resonance imaging shows restricted diffusion in both paramedian midbrain with hypointensity on apparent diffusion coefficient images. Initial examination showed almost complete supraduction paralysis bilaterally with torsional nystagmus (more prominent in the left eye). At rest, there was no significant deviation or misalignment of eyes. There was no downward gaze palsy, but saccadic eye movements appeared slightly impaired.
DISCUSSION
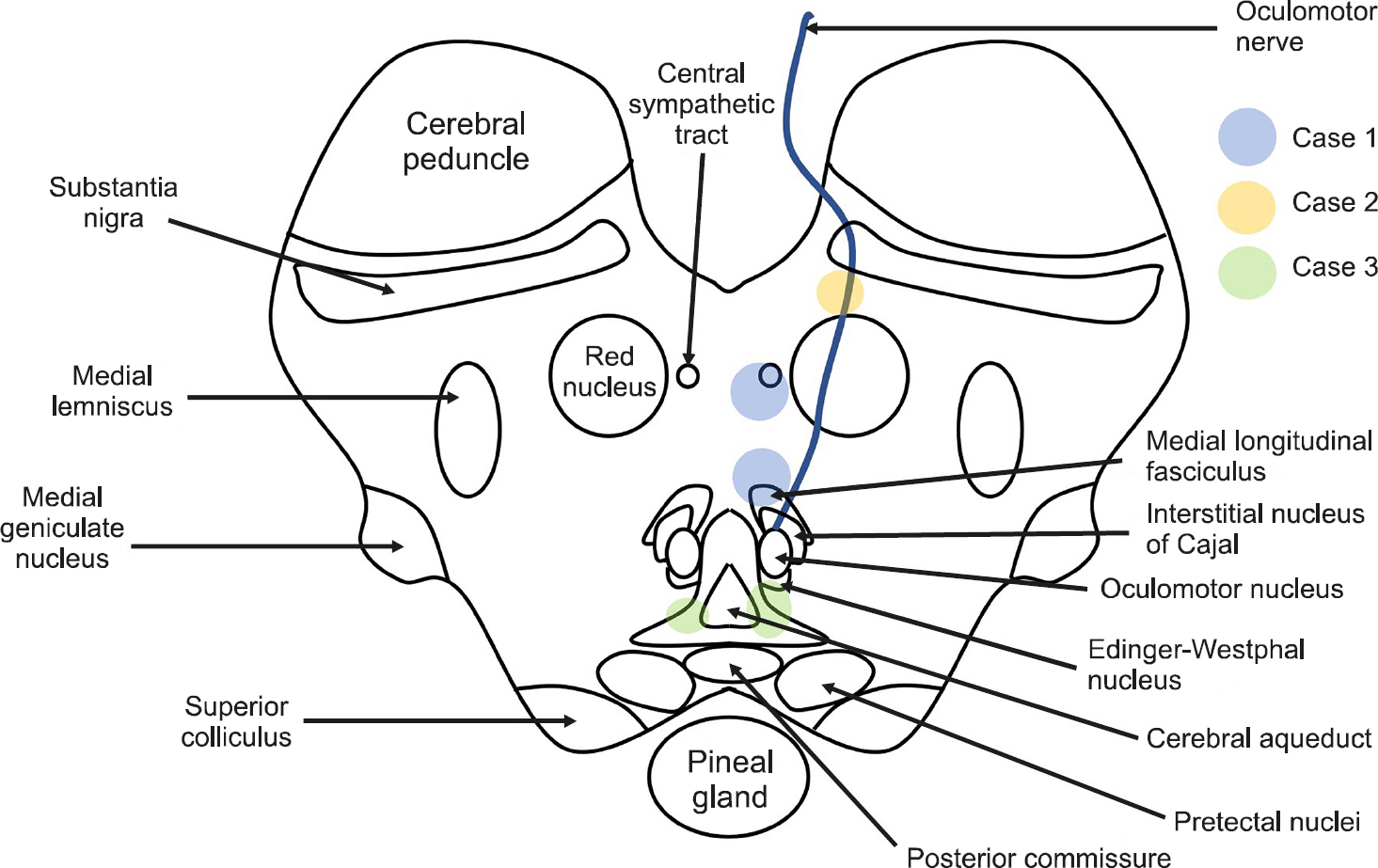
Because numerous ascending/descending tracts are gathered in the midbrain, small cerebral infarctions can result in various symptoms (Fig. 4). The most frequent clinical manifestation of lesions involving the midbrain is an eye movement disorder associated with a nuclear or fascicular origin [5]. This lesion contains the neural network for vertical eye movement, vergence, pupillary autonomic responses, eyelid movements, and the fasciculus involved in the horizontal gaze [1]. Thus, various eye movement abnormalities may be observed in lesions of the midbrain, but most of their clinical manifestations involve vertical eye movement abnormalities. Horizontal gaze palsy accompanied by Horner syndrome has not been reported in patients with midbrain infarction [2]. Our first patient had monocular adduction palsy with ptosis and miosis, although convergence was spared, suggesting a combination of INO and Horner syndrome. INO is characterized by impaired adduction of ipsilateral affected eye movement with abduction nystagmus of the contralateral eye [5]. INO is one of the most localized brainstem syndromes, resulting from a lesion in the medial longitudinal fasciculus (MLF) in the dorsomedial brainstem tegmentum of either the pons or the midbrain [1,6]. INO is distinct from medial rectus palsy, which is accompanied by other signs such as convergence sparing, dissociated vertical-torsional nystagmus, and coexistent OTR [4]. Our patient demonstrated preservation of convergence eye movements, which was suggestive of intact medial rectus innervation. Horner syndrome consists of a constellation of clinical findings, including the classic triad of unilateral ptosis, ipsilateral miosis with the normal pupillary response, and ipsilateral facial anhidrosis. This syndrome results from damage to the oculosympathetic pathways [7]. The combination of Horner syndrome and INO is extremely rare in a lesion of the mesencephalon. Ischemia of the paramedian dorsal midbrain at the superior colliculus level, ventral to the cerebral aqueduct (with fibers that track along with the MLF) could be involved [8]. However, the descending sympathetic tract in the midbrain runs dorsolaterally to the medial lemniscus. Therefore, it is challenging for the lesion to involve the MLF and the sympathetic tract simultaneously without other neurological abnormalities. Given the close proximity between these midbrain structures, the MLF is adjacent to the cerebral aqueduct, but the central sympathetic tract runs relatively adjacent to the ipsilateral spinothalamic tract that is laterally distant from the midline [9]. Considering the findings in our patient, the central sympathetic tract may be located on the medial side, rather than its location in the classical neuroanatomy paradigm. Although theoretically possible, pure INO with Horner syndrome has not been previously described in patients with midbrain lesions.
Fig. 4.Locations of midbrain lesions that produced the neurological manifestations in our patients.
Oculomotor nerve palsy is not a rare clinical syndrome. Usually, paralysis of the oculomotor nerve occurs due to the fascicular lesion composition of distinct syndromes corresponding to adjacent neural structures involved along the fascicular pathway through the red nucleus, substantia nigra, brachium conjunctivum, and crus cerebri [10]. However, isolated oculomotor nerve palsy without other neurological signs is a rare manifestation of midbrain infarction. In most patients described in the literature, the lesions have been located in the ventromedial part of the midbrain involving the oculomotor fascicles [2]. The midbrain infarct is commonly tiny in size due to partial fascicular involvement. The pupillary sphincter and inferior rectus muscles are commonly spared [11]. Our second patient showed isolated complete oculomotor nerve palsy with mydriasis in the left eye. MRI showed a lesion of the oculomotor nerve fascicle between the cerebral peduncle and the red nucleus in the ventromedial midbrain. Although most isolated complete third nerve palsy caused by a nuclear or fascicular lesion of the third nerve is accompanied by other neurological signs, our patient showed isolated complete third nerve palsy without additional signs.
The classic triad of Parinaud syndrome includes upward gaze palsy, pupillary light-near dissociation, and convergence-retraction nystagmus, similar to the findings in our patient [12]. The clinical features in our patient were identical to those of Parinaud syndrome, but the lesion location and etiology were far different.
Parinaud syndrome is a well-known complication of thalamic and pineal gland tumors or untreated hydrocephalus that causes extrinsic compressive lesions to the pretectal area and posterior commissure (PC) [1]. However, in our patient, all major dam-aged results from vascular ischemic lesions were located in the paramedian midbrain. Two separated small focus of both lesions was evident in the paramedian mesencephalon, which regards selectively involved the afferent/efferent fibers from the PC or nucleus of the PC. The clinical features described here have not reaches the bilateral lesion of the rostral interstitial nucleus of MLF (riMLF) or interstitial nucleus of Cajal (INC). The riMLF is located dorsomedial to the red nucleus and rostral to the oculomotor nuclei, which connection between burst neurons in riMLF to motoneurons innervating elevator muscles projected to be bilateral, but the depressor muscles projected to be ipsilateral [13]. This innervation predisposes midbrain lesions of the riMLF to provoked predominantly downward saccadic palsy [14]. Thus, axons fibers innervation might be crossing within the oculomotor nucleus and probably not through the PC.
The INC is the important neural integrator for vertical and torsional gaze located caudal to the riMLF [13]. It is known to conduct for the smooth vertical pursuit and vertical VOR. Although INC is located very close to riMLF, it may not have been included in the lesion in this case [3]. Because the patient's vertical VOR was preserved and there was no clinical evidence of OTR. If the lesion involving the INC, usually OTR needs to be accompanied due to the interruption of otolith-ocular projections to the INC [2]. Thus, this case shows that bilateral paramedian lesions were highly selectively involved in the neural fibers within the PC. Meanwhile, it is associated with a tiny, millimetric, and very circumscribed lesion that affects both upward gaze and probably pupillary reflexes. The patient had vertical gaze palsy, but the downward gaze was relatively preserved. Although, there is no clear separation between ter-ritories yielding upward and downward gaze. Nevertheless, the downward gaze projections were less affected due to the lesion location medially along with the riMLF; however, the fibers for upward gaze are projected more laterally, the upward gaze fibers are more predisposed to damage from surrounding lesions [15].
In summary, we have described rare neuro-ophthalmologic manifestations in patients with upper midbrain infarction. Notably, patients with midbrain infarction may present with specific signs and symptoms that are compatible with mesencephalic localization. The isolated or combined neuro-ophthalmologic signs and symptoms should be interpreted in the complex anatomical context described here and investigated by immediate brainstem neuroimaging analyses and careful neurologic examinations.
ARTICLE INFORMATION
-
No potential conflict of interest relevant to this article was reported.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by a research grant from Jeju National University Hospital in 2018 (201800360001).
REFERENCES
- 1. Lee SH, Kim HJ, Kim JS. Ocular motor dysfunction due to brainstem disorders. J Neuroophthalmol 2018;38:393–412.ArticlePubMed
- 2. Leigh RJ, Zee DS. The neurology of eye movements. 5th ed.Oxford: Oxford University Press; 2015.
- 3. Caminero F, Cascella M. Neuroanatomy, mesencephalon midbrain [updated 2020 Nov 2]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan.Available from:
https://www.ncbi.nlm.nih.gov/books/NBK551509/.
- 4. Vaphiades MS. Ocular motor dysfunction due to brainstem disorders. J Neuroophthalmol 2018;38:566. ArticlePubMed
- 5. Bogousslavsky J, Maeder P, Regli F, Meuli R. Pure midbrain infarction: clinical syndromes, MRI, and etiologic patterns. Neurology 1994;44:2032–40.ArticlePubMed
- 6. Atlas SW, Grossman RI, Savino PJ, Schatz NJ, Sergott RC, Bosley TM, et al. Internuclear ophthalmoplegia: MR-anatomic correlation. AJNR Am J Neuroradiol 1987;8:243–7.PubMedPMC
- 7. Giles CL, Henderson JW. Horner's syndrome: an analysis of 216 cases. Am J Ophthalmol 1958;46(3 Pt 1):289–96.ArticlePubMed
- 8. Kim JS, Kang JK, Lee SA, Lee MC. Isolated or predominant ocular motor nerve palsy as a manifestation of brain stem stroke. Stroke 1993;24:581–6.ArticlePubMed
- 9. Mossuto-Agatiello L. Caudal paramedian midbrain syndrome. Neurology 2006;66:1668–71.ArticlePubMed
- 10. Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology 2005;64:1227–32.ArticlePubMed
- 11. Amano Y, Kudo Y, Kikyo H, Imazeki R, Yamamoto M, Amari K, et al. Isolated unilateral oculomotor paresis in pure midbrain stroke. J Neurol Sci 2015;351:191–5.ArticlePubMed
- 12. Shields M, Sinkar S, Chan W, Crompton J. Parinaud syndrome: a 25-year (1991–2016) review of 40 consecutive adult cases. Acta Ophthalmol 2017;95:e792–3.ArticlePubMed
- 13. Bhidayasiri R, Plant GT, Leigh RJ. A hypothetical scheme for the brainstem control of vertical gaze. Neurology 2000;54:1985–93.ArticlePubMed
- 14. Jacobs L, Anders on PJ, Bender MB. The lesions producing paralysis of downward but not upward gaze. Arch Neurol 1973;28:319–23.ArticlePubMed
- 15. Serino J, Martins J, Páris L, Duarte A, Ribeiro I. Parinaud's syndrome due to an unilateral vascular ischemic lesion. Int Ophthalmol 2015;35:275–9.ArticlePubMed
Citations
Citations to this article as recorded by

- A Improved Case of Post Cerebral Infarction Dizziness and Gait Discomfort after Treated with Korean Medicine Treatment and Vestibular Rehabilitation Practice
Hongmin Chu, Hyeon-Seo Lim, Kwangho Kim, Young-Ung Lee, Kyungtae Park, Jongwon Jang, Ho-sun Ryu, Su-hak Kim, Cheol-hyun Kim, Sangkwan Lee, Kang-keyng Sung
Journal of Korean Medicine Rehabilitation.2020; 30(4): 179. CrossRef
 , 최재철, 김홍전, 강철후
, 최재철, 김홍전, 강철후  , Jay Chol Choi, Hong Jun Kim, Chul-Hoo Kang
, Jay Chol Choi, Hong Jun Kim, Chul-Hoo Kang 








 KBS
KBS

 PubReader
PubReader ePub Link
ePub Link Cite
Cite